Outcomes of Cytomegalovirus Status Determining Induction Therapy in Lung Transplant
K. Heagler1, J. Lyons1, B. Bemiss2, P. Stracener1
1Pharmacy, Loyola University Medical Center, Maywood, IL, 2Loyola University Medical Center, Maywood, IL
Meeting: 2021 American Transplant Congress
Abstract number: 98
Keywords: High-risk, Immunosuppression, Interleukin-2 receptor, Rejection
Topic: Clinical Science » Lung » Lung: All Topics
Session Information
Session Name: How to Expect the Unexpected- Incorporating Predictors into Lung Transplant Decision Making
Session Type: Rapid Fire Oral Abstract
Date: Saturday, June 5, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:35pm-6:40pm
Presentation Time: 6:35pm-6:40pm
Location: Virtual
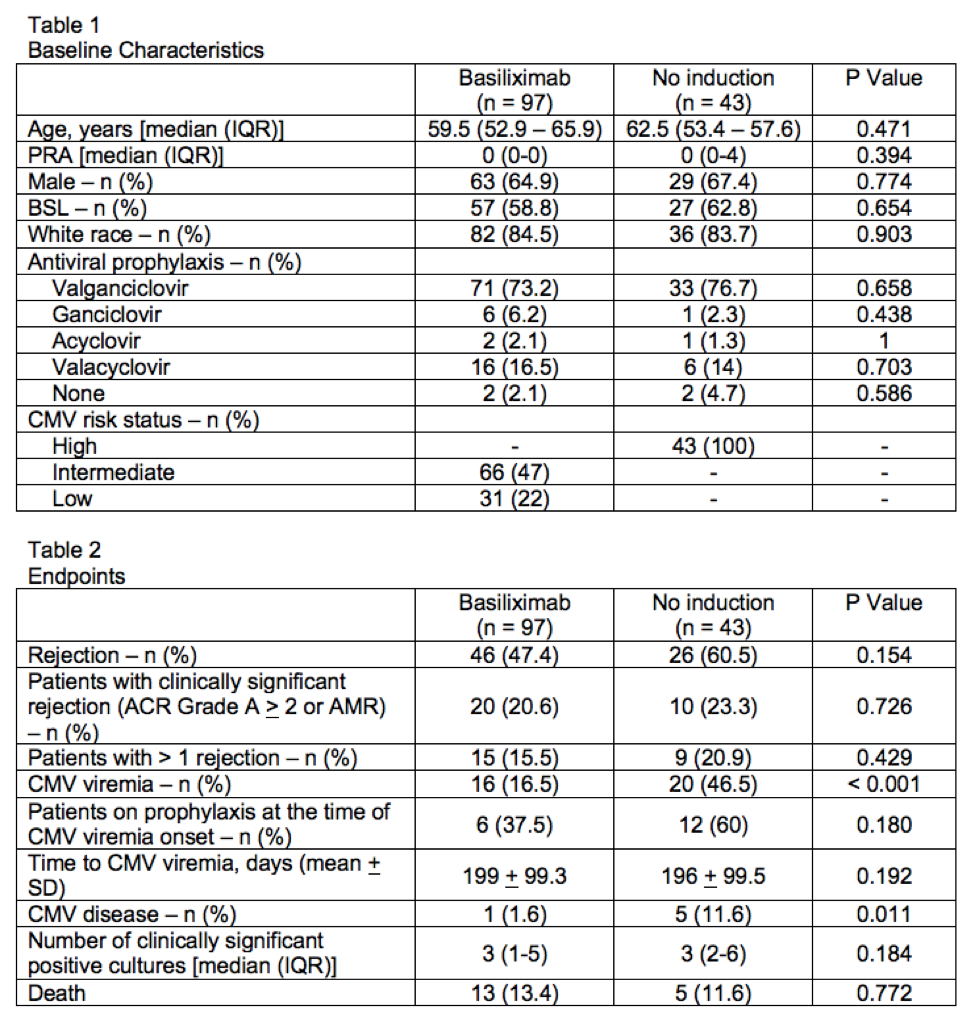
*Purpose: At the study institution, basiliximab induction is withheld in lung transplant recipients who are cytomegalovirus (CMV) high risk due to concern for increased CMV infection. The purpose of this study was to assess the difference in rate of rejection and CMV infection between basiliximab induction compared to no induction in lung transplant recipients.
*Methods: This retrospective cohort study compared adult lung transplant recipients who received basiliximab induction compared to no induction between January 1, 2015 and November 30, 2019. The primary endpoint was incidence of rejection within the first-year post-transplant. Secondary endpoints included patients with clinically significant rejection (ACR Grade A > 2 or AMR), patients with greater than one rejection, incidence of CMV viremia, CMV disease, number of clinically significant positive cultures, and death at one-year post-transplant.
*Results: A total of 140 patients were included, 97 in the basiliximab group and 43 in the no induction group. Baseline characteristics were similar between the groups as shown in Table 1. Median age was 60 years, 66% were male, and 60% received a bilateral sequential lung transplant (BSL). Primary and secondary endpoint results are displayed in Table 2. Incidence of rejection was numerically higher in the no induction group 26/43 (60.5%) compared to the basiliximab group 46/97 (47.4%) (P = 0.154). Rejection type and severity were similar between the two groups. A significantly higher number of patients had CMV viremia and disease in the no induction group compared to the basiliximab group. There was no difference between the groups in all other secondary endpoints.
*Conclusions: Withholding induction therapy in lung transplant recipients due to CMV high risk status resulted in a numerically higher incidence of rejection and did not significantly impact the total number of infections. CMV viremia and disease was significantly higher in the no induction group, which is an expected finding given their CMV high risk status. Notably, a large number of patients in each group were not on prophylaxis at the time of viremia onset. These findings do not provide strong evidence to justify withholding induction based on CMV risk status. A study of direct comparison including CMV high risk patients receiving basiliximab versus no induction would be needed to better assess the impact of induction therapy on the incidence of CMV viremia and disease after lung transplant.
To cite this abstract in AMA style:
Heagler K, Lyons J, Bemiss B, Stracener P. Outcomes of Cytomegalovirus Status Determining Induction Therapy in Lung Transplant [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-cytomegalovirus-status-determining-induction-therapy-in-lung-transplant/. Accessed February 24, 2026.« Back to 2021 American Transplant Congress