Outcomes of Antiviral Treatment (AVT) in Hepatitis C Virus (HCV) Liver Transplant (LT) Patients off Immunosuppression (IS) in Direct Acting Antivirals (DAA) Era
E. Agudelo,1,2 I. Campos-Varela,3 N. Terrault.1,2
1Medicine, University of California San Francisco, San Francisco, CA
2Surgery, University of California San Francisco, San Francisco, CA
3Internal Medicine, Hospital of Santiago de Compostela, Santiago de Compostela, Spain.
Meeting: 2018 American Transplant Congress
Abstract number: D246
Keywords: Hepatitis C, Liver transplantation, Recurrence
Session Information
Session Name: Poster Session D: Liver: Viral Hepatitis
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Clearance of HCV under AVT, including DAAs, has been associated with higher risk of rejection, possibly due to the changing immunological environment within the liver. Whether patients who are not on IS during antiviral therapy are at higher risk of rejection is unknown.
Aim: To describe the outcomes of LT patients who are off IS and subsequently treated with DAA therapy.
Methods: All HCV infected patients off IS who received AVT were identified. For each identified patient, 1 control receiving regular IS and matched by date of AVT and duration since LT was compared.
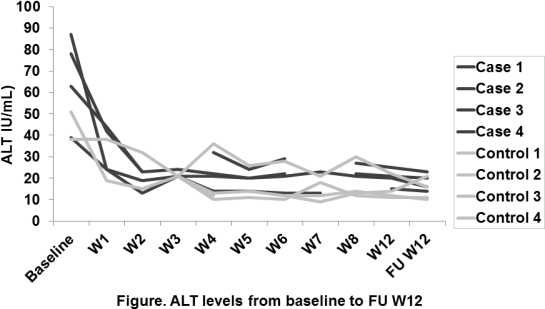
Results: Between May and December 2016, 4 patients who received AVT and were off IS were identified. Cases and controls were all men, with median age 65 and 60 yrs, with HCV genotype 1; patients were treated for 12 weeks with SOF/LDV plus ribavirin (RBV) (n=8). Liver biopsy done closest to the time of HCV treatment showed Stage 0(N=3)-1(N=1) in cases and Stage 1(N=2)-2(N=2) in controls. At the time of AVT, patients were off IS for 4.6 yrs, 5.6 yrs, 13.4 yrs and 16.0 yrs. Time from LT to AVT was 12.9 yrs (range,7.5-23.4) and 5.7 yrs (range,4.4-20.9) for cases and controls respectively. Median viral load was higher in patients receiving IS 5.5 log IU/mL (range,4.9-5.7) vs. 6.3 log IU/mL (range,6.1-7.0). Median baseline ALT was 70 U/L (IQR 51-82) and 35 U/L (IQR 22-44) for cases and controls and at follow-up w12 was 18 U/L (IQR 15-21) and 13 U/L (IQR 8-18), [Figure]. Similar trends were seen with AST. No signs of rejection were observed during AVT or first 3 months of follow-up after the end of therapy. All 8 patients obtained sustained virological response.
Conclusions: DAA therapy in HCV patients who have been weaned off IS post-LT shows that treatment can be undertaken without need to restart IS and with SVR achieved without immunologic complications. Confirmation in a larger series is warranted but these preliminary results are encouraging.
CITATION INFORMATION: Agudelo E., Campos-Varela I., Terrault N. Outcomes of Antiviral Treatment (AVT) in Hepatitis C Virus (HCV) Liver Transplant (LT) Patients off Immunosuppression (IS) in Direct Acting Antivirals (DAA) Era Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Agudelo E, Campos-Varela I, Terrault N. Outcomes of Antiviral Treatment (AVT) in Hepatitis C Virus (HCV) Liver Transplant (LT) Patients off Immunosuppression (IS) in Direct Acting Antivirals (DAA) Era [abstract]. https://atcmeetingabstracts.com/abstract/outcomes-of-antiviral-treatment-avt-in-hepatitis-c-virus-hcv-liver-transplant-lt-patients-off-immunosuppression-is-in-direct-acting-antivirals-daa-era/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress