Outcomes of ABO Incompatible Transplant on Steroid Free Immunosuppression
Department of Medicine, Division of Nephrology, Indiana University School of Medicine, Indianapolis, IN
Meeting: 2019 American Transplant Congress
Abstract number: A254
Keywords: Graft survival, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: ABO incompatible (ABOi) kidney transplant (KTx) was introduced to expand donor organ pool. However it is also an immunologically high-risk procedure requiring pre-transplant antibody depletion as well as maintenance on robust post-transplant immunosuppression. Currently data regarding outcomes of ABOi KTx on steriod-free immunosuppression is scarce. Beginning in 2003, we have utilized steriod-free maintenance immunosuppression regimen. Our aim is to determine the impact of such regimen in ABOi KTx recipients on graft and patient survival 1-year post transplant.
*Methods: Retrospective review of 43 patients receiving ABOi KTx between January 2005 and September 2017 at an academic transplant center with mean follow up of 3.5+/-2.8 years was performed. Patients with less than 1-year follow up were excluded. All patients received peri-transplant antibody depleting therapies (plasmapheresis (PP), IVIG, rituximab (RTX)) as indicated by anti-A titers, along with anti-thymocyte globulin with steroid induction followed by rapid taper (5 days), then maintained on tacrolimus and mycophenolate.
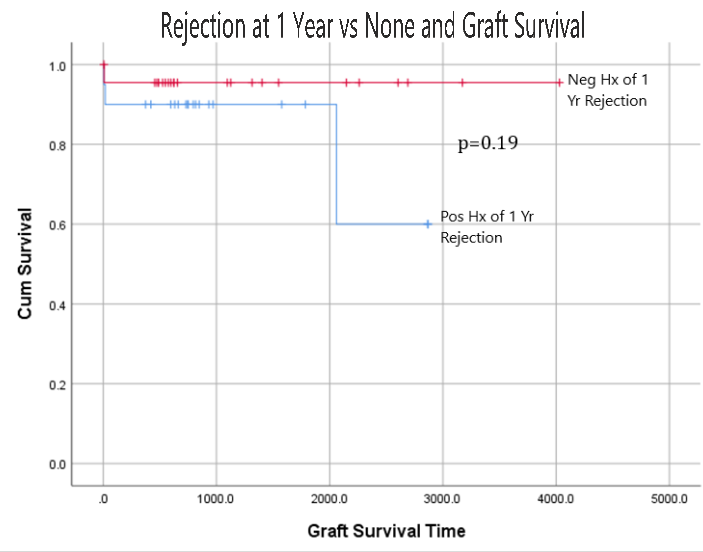
*Results: Mean age at KTx was 51+/-14 years (72% Caucasian and 69.8% male). 65% were A2 donor to O recipient, while 27% are A2 to B, 4% are A2B to B, and 2% are A1 to O. 31 patients received PP only, 4 received PP+IVIG, 2 received PP+RTX, 2 received RTX alone, and 4 received all three. 2 patients remained on steroid 1-year post transplant. Rate of 1-year rejection (both acute cellular and mixed rejections) was 46.5%. No significant difference (p=0.72) was found in the incidence of 1-year rejection irrespective of peri-transplant antibody depleting therapy received. 1, 3, and 5-year graft survival were 93%. Mean GFR at 1, 3, and 5 years were 58.9, 59 and 52 ml/min, respectively. No significant difference was found in graft survival irrespective of rejection status (p=0.19) or type of peri-transplant antibody depleting therapy (p=0.7). Patient survival over study period was 93% and was neither affected by history of acute rejection (p=0.19) nor by peri-transplant antibody depleting therapies (p=0.71).
*Conclusions: Although steroid-free maintenance immunosuppression regimen is associated with high risk of 1-year acute rejection in ABOi KTx recipients, it has no significant impact on graft or patient survival. Interestingly, addition of IVIG+/-RTX to PP does not appear to provide added graft or patient survival benefit in our study population. Further studies are needed to determine the optimal antibody depleting therapy in ABOi KTx transplant recipients.
To cite this abstract in AMA style:
Wang A, Mishler D, Taber T, Yaqub M, Sharfuddin A, Goggins W, Adebiyi O. Outcomes of ABO Incompatible Transplant on Steroid Free Immunosuppression [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-abo-incompatible-transplant-on-steroid-free-immunosuppression/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress