Outcome Implications of Prescription Opioid and Benzodiazepine in Lung Transplant Patients: Analysis of Linked Registry and Pharmacy Claims Data
1Department of Medicine, Saint Louis University, Saint Louis, MO, 2Univ of New Mexico, Albuquerque, NM, 3Univ of Virginia, Charlottesville, VA, 4Univ of Pittsburgh, Pittsburgh, PA, 5Johns Hopkins, Baltimore, MD, 6Univ of Iowa, Iowa City, IA, 7Drexel University, Philadelphia, PA, 8SRTR, Minneapolis, MN, 9Saint Louis University, Saint Louis, MO
Meeting: 2020 American Transplant Congress
Abstract number: 189
Keywords: Lung transplantation, Mortality, Outcome, Risk factors
Session Information
Session Name: Living in the Real World: Decision Making and Outcomes After Lung Transplant
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: To investigate the frequency, correlates, and outcomes associated with prescription opioid and benzodiazepine use before and after lung transplant (LuTx).
*Methods: We examined a linkage of Scientific Registry of Transplant Recipients data with records from a pharmaceutical claims warehouse to characterize prescription opioid and benzodiazepine use in the years before and after LuTx. Associations (adjusted hazard ratio, LCLaHRUCL) with death were examined by Cox regression, adjusted for baseline patient, donor, and transplant factors.
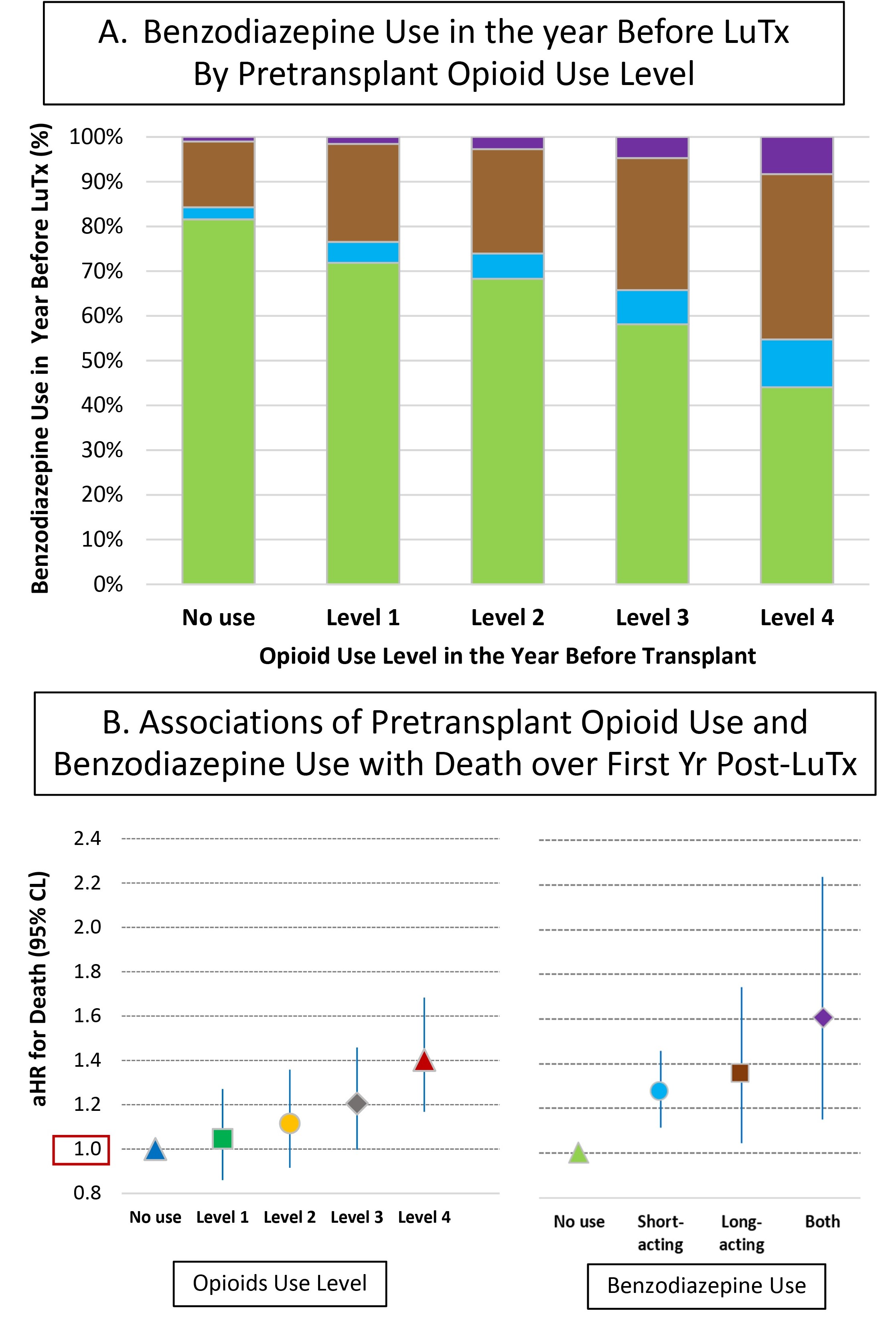
*Results: Among 11,568 LuTx recipients (2008-2017), 33.7% filled an opioid prescription in the year before transplant; use was more common among those who were younger, black or Hispanic, less than college educated, or on dialysis, extracorporeal membrane oxygenation, or ventilator support. Benzodiazepine use was higher among those who also filled opioids: 28% among those without prescription opioid use; 56% among those with the highest level opioid use (>2000 annual morphine equivalents) (Fig 1A). Pre-LuTx opioid use was associated with a graded increase in mortality over the first year post-LuTx (Fig 1B), with a 40% mortality increase for those with highest use (aHR, 1.191.401.75). Benzodiazepine use was also associated with increased mortality, with 60% increased risk for those who filled both short- and long-acting (aHR, 1.181.602.22). Independent effects were persistent when opioids and benzos were co-entered in the same model, but interactions were not observed. Similar risk relationships occurred for prescription use in the year after LuTx, such that the highest level opioid use (aHR, 1.551.761.89), and use of both short- and long-acting benzodiazepines (aHR, 1.241.641.92), were each associated with increased mortality over >1-5 years.
*Conclusions: While associations may, in part, reflect underlying conditions or behaviors, prescription opioid and benzodiazepine use history is relevant in assessing and monitoring LuTx candidates and recipients. Future research is needed to examine mechanisms of these associations, and to develop strategies to improve outcomes.
To cite this abstract in AMA style:
Lentine K, Myaskovsky L, Bruschwein H, Dew M, Zhang Z, Demarco MMcAdams, Segev D, Axelrod D, Hess G, Kasiske B, Snyder J, Schnitzler M. Outcome Implications of Prescription Opioid and Benzodiazepine in Lung Transplant Patients: Analysis of Linked Registry and Pharmacy Claims Data [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/outcome-implications-of-prescription-opioid-and-benzodiazepine-in-lung-transplant-patients-analysis-of-linked-registry-and-pharmacy-claims-data/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress