Novel Oral Anticoagulants in Kidney Transplantation: Results from a Pilote Study
J. Leon, J. Zuber, L. Amrouche, D. Anglicheau, G. Divard, C. Legendre, R. Sberro-Soussan
Adult Transplantation, Necker Hospital, Paris, France
Meeting: 2019 American Transplant Congress
Abstract number: C196
Keywords: Anticoagulation, Kidney transplantation, Safety, Tolerance
Session Information
Session Name: Poster Session C: Kidney: Cardiovascular and Metabolic
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
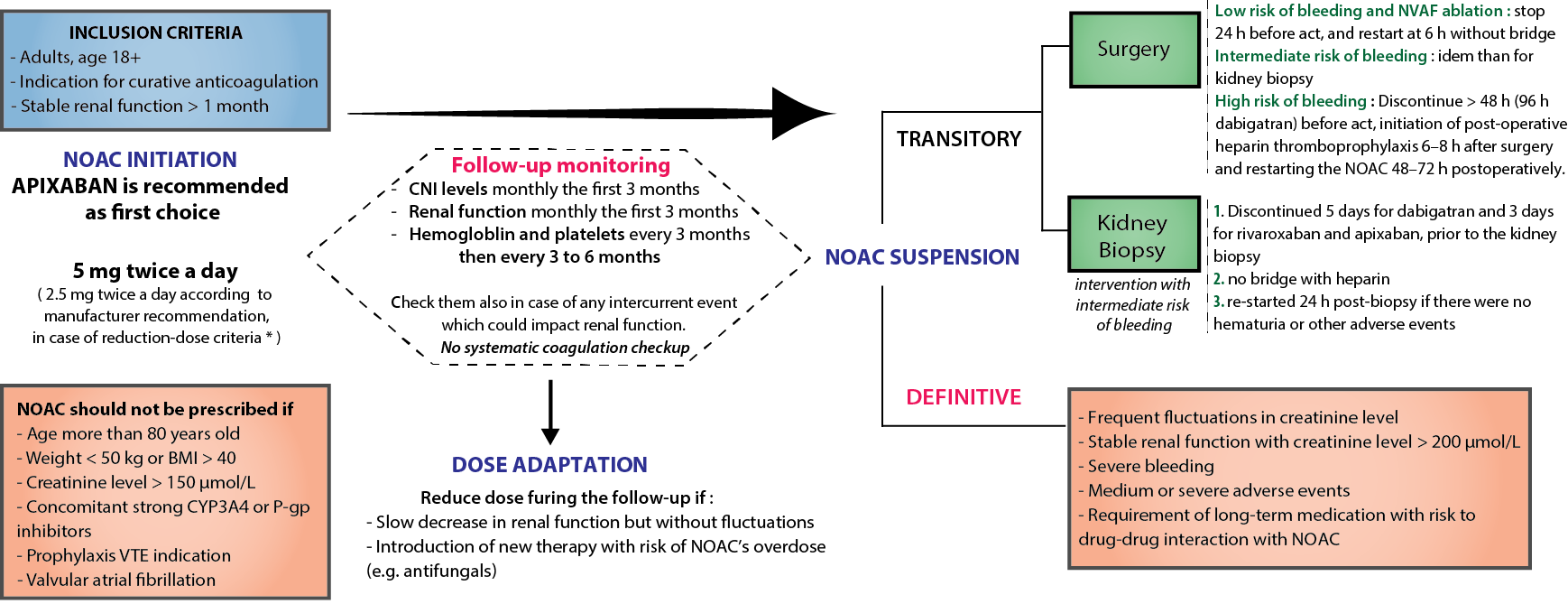
*Purpose: Oral anticoagulation therapy is frequently prescribed to kidney transplant recipients, for prevention and treatment of thrombotic events. Over the past ten years, novel oral anticoagulants (NOACs) have shown similar efficacy with safety profiles equal or superior to those of vitamin K antagonists in the general population. However, only few data are available regarding the safety and efficacy of NOACs in kidney transplantation.
*Methods: This pilote single-center retrospective study included all kidney transplant recipients that have received NOACs for more than one month. Patients older than 80 years and weighing less than 50 kg were excluded. Thrombotic events, hemorrhagic complications and allograft outcomes were reported.
*Results: From 2013 to 2018, 33 kidney transplant recipients started NOAC therapy at a median of 54 months after transplantation. Patients were 42% women, with a median age of 64 [50-71] years old and a median renal function of 56 mL/min/1.73 m2. The major indication was non-valvular atrial fibrillation (58%), followed by venous thromboembolic disease (21%). Apixaban was the most commonly used agent (67%), associated in more than 90% of cases with calcineurin inhibitors, as part of their concomitant immunosuppressive treatment. No thrombotic complications were reported through the final follow-up (median 13 [6-20.5] months). Four patients (12%) experienced minor bleeding events, which didn’t require transfusion or reversal agents. No significant changes in kidney function, hemoglobin levels or tacrolimus doses were reported.
*Conclusions: NOACs seemed to be effective and safe anticoagulant treatments in a subset of selected kidney transplant recipients with stable renal function. A randomized clinical trial is required to confirm these results.
To cite this abstract in AMA style:
Leon J, Zuber J, Amrouche L, Anglicheau D, Divard G, Legendre C, Sberro-Soussan R. Novel Oral Anticoagulants in Kidney Transplantation: Results from a Pilote Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/novel-oral-anticoagulants-in-kidney-transplantation-results-from-a-pilote-study/. Accessed March 7, 2026.« Back to 2019 American Transplant Congress