No Significant Outcome Benefit for Brand-Name Versus Generic Tacrolimus in Kidney Transplant Recipients: SRTR Analysis
1Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH
2Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH.
Meeting: 2015 American Transplant Congress
Abstract number: 67
Keywords: Graft survival, Immunosuppression, Kidney transplantation, Outcome
Session Information
Session Name: Concurrent Session: Regulatory Issues in Transplant Administration
Session Type: Concurrent Session
Date: Sunday, May 3, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Room 118-C
Intro
Generic formulations of tacrolimus have been available for maintenance immunosuppression since 2009. Our aim was to evaluate the frequency of use of generic tacrolimus and compare outcomes with the brand-name formulation.
Methods
We identified solitary kidney transplant recipients in the SRTR database discharged on either brand-name tacrolimus(BTac) or generic tacrolimus(GTac) between 2010-2013(n=58,036). We compared transplant characteristics, acute rejection rates, one-year renal function and graft and patient survival using univariate and multivariable models.
Results
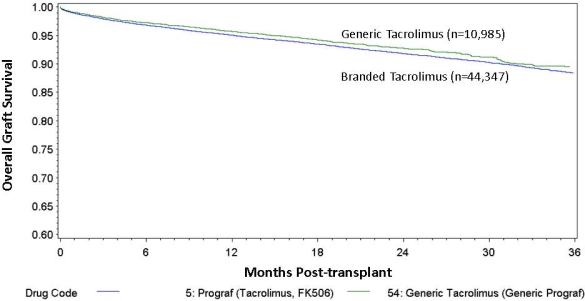
Overall, 20% of the study population were on generic tacrolimus increasing from 11% to 30% from 2010-2013. Average recipient and donor age were similar between groups (49.6 yrs/39.2 yrs for BTac) and (49.9 yrs/38.4 yrs for GTac) respectively. BTac was associated with a higher proportion of living donor transplants (36% vs 31%, p<0.001) and black recipients (26.4% vs 21.7%, p<0.001), and a lower proportion of patients with diabetes as primary diagnosis (24.2% vs 25.6%, p=0.002). Overall graft survival at 1 and 3 years was slightly higher among patients on GTac (96% and 89%) compared to BTac(95% and 88%, Figure1, p=0.01). After risk adjustment, GTac was associated with lower hazard for overall graft loss(AHR=0.87, 95% C.I. 0.78-0.96) and death(AHR=0.82, 95% C.I. 0.71-0.93). One-year acute rejection rates were slightly higher among recipients on GTAc (8.1% vs 7.3%, p=0.02). One-year treatment for BK virus was similar (7.2% for GTac, 6.6% for BTac, p=0.07) and one-year hospitalizations were higher among patients on BTac (39.4% vs 35.3%, p<0.001). One-year average eGFR was similar between groups (67.8 mL/min for BTac and 67.3 mL/min for GTac, p=0.44).
Conclusion
While there may be variation in outcomes between different generic formulations, there is no evidence of significant adverse outcomes for kidney transplant recipients with use of generic tacrolimus.
[emsp]
Figure 1. Overall Graft Survival by Brand-Name versus Generic Tacrolimus (p=0.01)
To cite this abstract in AMA style:
Goldfarb D, Poggio E, Flechner S, Fatica R, Schold J. No Significant Outcome Benefit for Brand-Name Versus Generic Tacrolimus in Kidney Transplant Recipients: SRTR Analysis [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/no-significant-outcome-benefit-for-brand-name-versus-generic-tacrolimus-in-kidney-transplant-recipients-srtr-analysis/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
