Monoclonal Antibody Which Possibly May Replace the Therapeutic Intravenous Immunoglobulin, A
HLA Antibodies, Terasaki Foundation Laboratory, Los Angeles, CA
Meeting: 2013 American Transplant Congress
Abstract number: D1512
The value of intravenous immunoglobulin (IVIg) in lowering HLA antibodies (Abs) has recently come into question. High variability in different therapeutic preparations may account for the variable outcomes. We have produced a monoclonal Ab (mAb) which mimic immunoreactivity and immunosuppressive capabilities of IVIg and provide a stable reliable replacement for current IVIg.
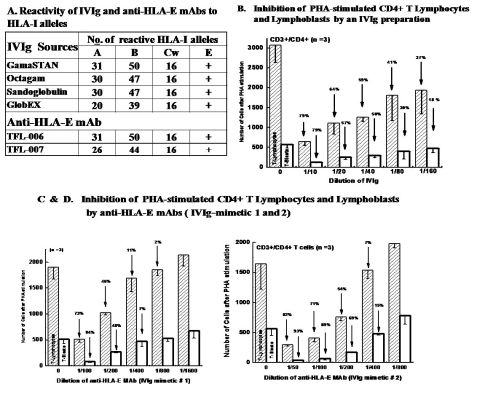
Four IVIg preparations were tested for their reactivity of HLA class-Ia and Ib alleles, and shown to have high reactivity to HLA class-I alleles (high mean fluorescent intensity and titers up to 1/512, by Luminex microbead testing) and to HLA-E. The numbers of HLA class-Ia allelic reactivities of IVIg preparations are shown in A. Since the blood of healthy individuals was shown to have HLA-Ia IgG Abs, the concentration of IgG from the plasma pooled from of donors accounts for the high levels of anti-HLA-Ia reactivities. Importantly, depleting anti-HLA-E Abs from IVIg totally eliminated the HLA-Ia reactivity of IVIg. We accordingly developed mAbs against recombinant HLA-E, which had reactivity to HLA class-I alleles identical to the different preparations of IVIg (A). Inhibition of the antibody reactivities with synthetic peptides showed that HLA-E shares epitopes with HLA-Ia alleles.
The immunosuppressive functions of IVIg were then compared among different preparations by suppression of PHA-activated proliferation of CD4+ T cells with that of the two anti-HLA-E mAbs. Carboxyfluorescein succinimidyl ester (CFSE) staining was used to monitor proliferation of T cells after different treatments. A striking similarity was noted between different preparations of IVIg (Figure B) and the anti HLA E mAbs (Figures C and D) in the dose dependent inhibition of PHA-stimulated blastogenesis of CD4+ T cells.
Since CD4+ T cells are involved in donor specific antigen presentation in allograft recipients for production of plasma B cells, inhibition of their proliferation and blastogenesis may account for their ability to reduce antibodies. We conclude that our unique anti-HLA-E mAb have the potential to replace the current IVIg, pooled and purified from the plasma of thousands donors.
Terasaki, P.: Stockholder, One Lambda, Inc.
To cite this abstract in AMA style:
Ravindranath M, Terasaki P. Monoclonal Antibody Which Possibly May Replace the Therapeutic Intravenous Immunoglobulin, A [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/monoclonal-antibody-which-possibly-may-replace-the-therapeutic-intravenous-immunoglobulin-a/. Accessed June 30, 2025.« Back to 2013 American Transplant Congress
