Meta-Analysis of the Chronic Exposure to Calcineurin Inhibitors and the Risk for Chronic and End-Stage Kidney Disease in Non-Renal Solid Organ Transplant Recipients
1Hospital for Sick Children, Toronto, ON, Canada, 2Royal Hospital for Children, Glasgow, United Kingdom, 3St. Michael’s Hospital, Toronto, ON, Canada, 4University of Toronto, Toronto, ON, Canada, 5Mount Sinai Hospital, Toronto, ON, Canada, 6E. M. Uleryk Consulting, Toronto, ON, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 1703
Keywords: Calcineurin, Nephrotoxicity, Renal dysfunction, Renal failure
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Calcineurin inhibitors (CNI) are standard of care immunosuppressants among all solid organ transplant (SOT) recipients, and the most common side effect is nephrotoxicity. There is little evidence on CNI dose, level or duration of cumulative exposure associated with chronic and end-stage kidney disease (CKD/ESKD). We conducted a systematic review and meta-analysis of chronic CNI exposure associated risk to CKD/ESKD in non-renal SOT recipients.
*Methods: Search terms included exposure to tacrolimus or cyclosporine among non-renal SOT recipients and CKD and ESKD as the primary outcome in MEDLINE, EMBASE, Cochrane and grey literature from inception to April 2021. Publications were reviewed by two independent reviewers. We categorized exposure by cyclosporine vs. tacrolimus and center based CNI standard of care vs. CNI sparing strategies. We assessed risk of bias for the quality of studies according to Newcastle Ottawa Scale, Cochrane Risk of Bias tool for Randomized Trials, and the National Institute of Health tool for Cross-Sectional Studies. Pooled results used a random-effects model according to exposure and study design.
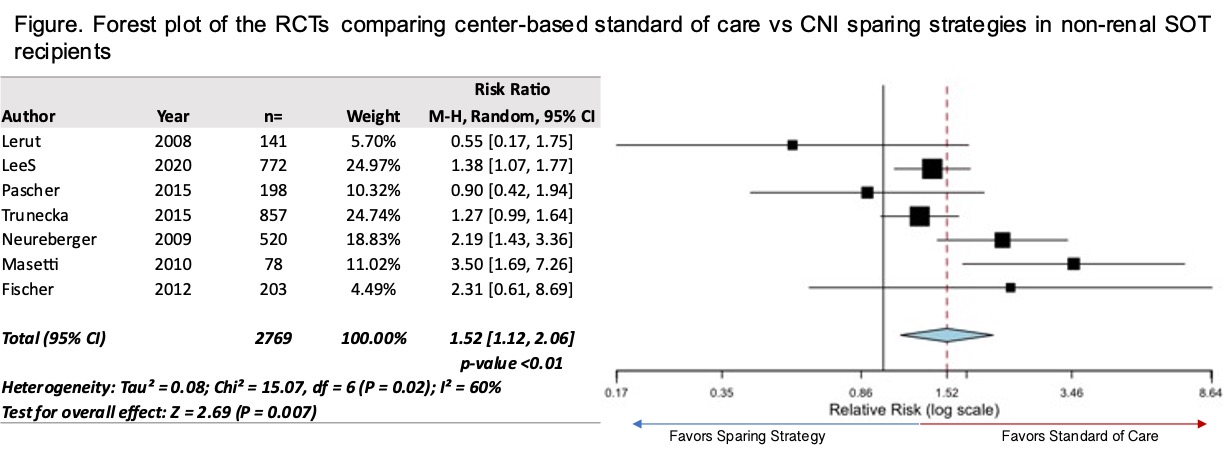
*Results: In 47 observational studies (4 in children) and 10 randomized controlled trials (RCTs) that met inclusion criteria, there were 20,2082 recipients (31% female) with an average follow-up of 5.7 years and mean age at transplantation of 49 years. Risk of bias assessment was low in 22% of observational studies and 38% of RCTs. There was no consistent definition of CNI trough level, dose, or duration, which led to heterogeneity among studies. Those on cyclosporine had 1.41 higher odds of CKD/ESKD compared to those on tacrolimus (1.11-1.80 95%CI). There is a 1.52 increased risk of CKD/ESRD in RCTs among those on standard CNI than sparing strategy (1.12-2.06 95%CI; Figure).
*Conclusions: Use of cyclosporine and conventional CNI dosing is associated with an increased risk of CKD/ESKD, however, we could not show a dose-response curve for increased risk due to the heterogeneity of exposure of CNI in dose, levels, or cumulative exposure. Increased risk of CNI needs to be further characterised to address chronic CNI exposure to ultimately mitigate risk for CKD.
To cite this abstract in AMA style:
Elias AAlvarez, Riedl M, Stewart D, Ruco A, Bobos P, Maharaj S, Shah V, Uleryk E, Teoh C, Parekh R. Meta-Analysis of the Chronic Exposure to Calcineurin Inhibitors and the Risk for Chronic and End-Stage Kidney Disease in Non-Renal Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/meta-analysis-of-the-chronic-exposure-to-calcineurin-inhibitors-and-the-risk-for-chronic-and-end-stage-kidney-disease-in-non-renal-solid-organ-transplant-recipients/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress