Longitudinal Surveillance, Relative Change Value (RCV) Improve Diagnostic Test Accuracy (DTA) of Donor-Derived Cell Free DNA (dd-cfDNA)
Intermountain Medical Center, Murray, UT
Meeting: 2021 American Transplant Congress
Abstract number: 629
Keywords: Rejection
Topic: Clinical Science » Biomarkers, Immune Assessment and Clinical Outcomes
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: dd-cfDNA, an injury biomarker in kidney transplant (KTx), is emerging as a trigger for biopsy (Bx) and superior to the traditional trigger of change in renal function. This is based on single event/Bx bracketing measurement of dd-cfDNA. Rational interpretation of serial dd-cfDNA, as an injury biomarker demands assessment of variability (intra- and Interindividual) and RCV of dd-cfDNA. We hypothesized that incorporating RCV vs. single cutoff value improves DTA of dd-cfDNA for rejection.
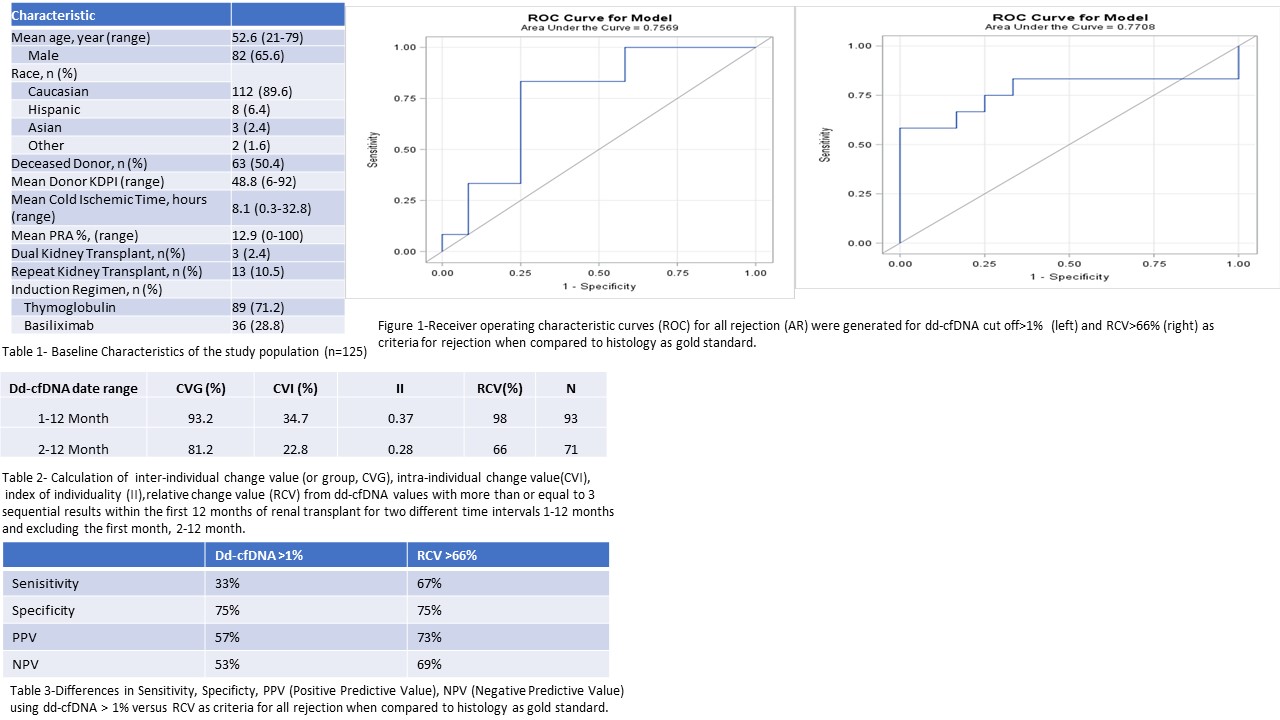
*Methods: After excluding patients with biopsy proven acute rejection and CMV/BK viremia, patients with serial dd-cfDNA at 1,2,3,4, 6, 9, 12-months post Tx were included. We computed the intra-individual change value(CVI), inter-individual or group change value (CVG), from dd-cfDNA values with more than or equal to 3 sequential results within the first 12 months of renal transplant. We also calculated index of individuality (II) as the CVI/CVG ratio and the relative change value (RCV). RCV is defined as the difference that must be exceeded between 2 sequential results for a significant change to occur. In a subset analysis of 24 patients with rejection, receiver operating characteristic curves (ROC) for all rejection (AR) were generated for dd-cfDNA cut off>1% and RCV>66% as criteria for rejection when compared to histology as gold standard.
*Results: Of 125 patients, 82 (65.6%) participants were male, with a mean age of 52.6 (Table 1).CVI was 34.7%, CVG was 93.2%., II was calculated at 0.37, and RCV was 98% (Table 2). After excluding one month values, due to largely higher values at one month in part of ischemia reperfusion injury, RCV changed to 66%. For dd-cfDNA>1%, discrimination for all rejection (AR), AUC was 0.7569 versus 0.7708 when compared to RCV >66% as criteria (Fig 1). Utilizing RCV as a criteria resulted in increase of 28% and 21% for positive predictive value (PPV) and negative predictive value (NPV) respectively ( Table 3).
*Conclusions: 1) Our study reports performance of RCV in ‘real world’ stable KTx population outside of a clinical trial. 2) An RCV of 66% in ‘real world’ Ktx population is similar to clinical trial results of 61%. We propose:1) Longitudinal surveillance of dd-cfDNA and RCV should supplant a single cutoff value for dd-cfDNA at biopsy 2) Clinical decision making using dd-cfDNA should be personalized at the patient level, taking into account both baseline values and longitudinal intra-individual trends.
To cite this abstract in AMA style:
Anand S, Lopez-Verdugo F, Sanchez-Garcia J, Dong L, Fife M, Krong J, Morris D, Srinivas T. Longitudinal Surveillance, Relative Change Value (RCV) Improve Diagnostic Test Accuracy (DTA) of Donor-Derived Cell Free DNA (dd-cfDNA) [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/longitudinal-surveillance-relative-change-value-rcv-improve-diagnostic-test-accuracy-dta-of-donor-derived-cell-free-dna-dd-cfdna/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress