Longer-Term Outcomes from the THINKER and EXPANDER Trials of Transplantation Using HCV-Viremic Kidneys
V. S. Potluri1, F. Naqvi2, P. Reese1, M. Shah1, D. Brown2, S. Prenner1, A. Loupy3, A. Woodards1, P. Abt1, E. Blumberg1, J. Trofe-Clark1, D. Segev2, R. Bloom1, D. Sawinski1, C. Durand2, P. Porrett1, M. Levine1, D. Goldberg4, N. Desai2
1University of Pennsylvania, Philadelphia, PA, 2Johns Hopkins, Baltimore, MD, 3Paris Transplant Group, Paris, France, 4University of Miami, Miami, FL
Meeting: 2020 American Transplant Congress
Abstract number: 282
Keywords: Cadaveric organs, Hepatitis C, Kidney transplantation, Viral therapy
Session Information
Session Name: All Organs: Viral Hepatitis
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: The practice of transplanting HCV-viremic kidneys into HCV-negative recipients has become more common, but longer-term outcomes have not been reported. We present data on kidney transplant recipients from the THINKER (NCT02743897) and EXPANDER (NCT02781649) trials, which launched in the year 2016.
*Methods: In EXPANDER, 10 recipients received elbasvir-grazoprevir just prior to transplantation and DAA therapy was tailored as needed after viral genotyping. For the 35 THINKER recipients, only HCV genotype 1 or 4 donor organs were accepted and elbasvir-grazoprevir therapy was started on day 3 post-transplant. Using a multi-dimensional prediction score (iBox) we estimate the projected long-term allograft survival.
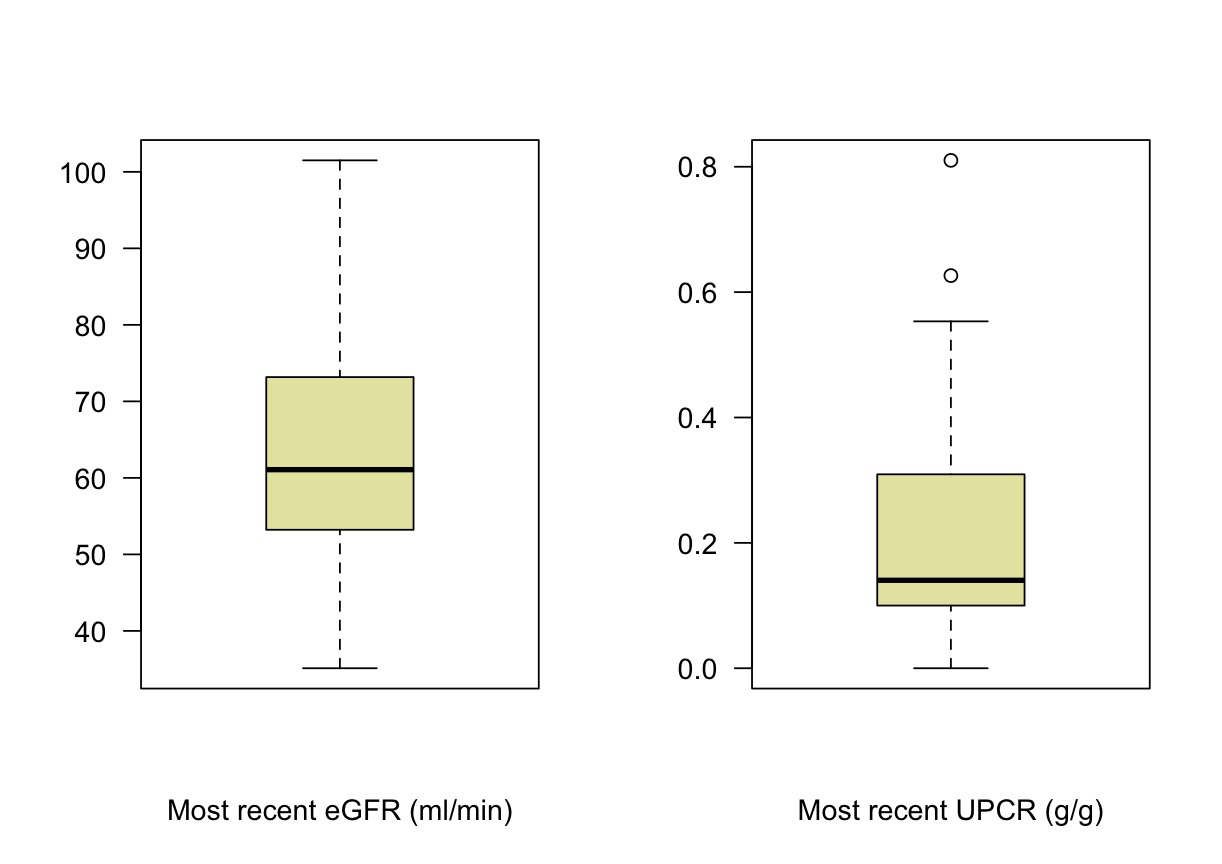
*Results: The median age at transplant was 63 years and 13 recipients (29%) were black race. The median time to transplant from initial wait-listing was 342 days [IQR 166-521 days]. All patients achieved SVR-12. Twelve recipients (27%) had delayed graft function. One recipient experienced rejection and none had primary graft failure. One THINKER recipient died two years post-transplant of adenocarcinoma considered unrelated to HCV or its therapy. Eight patients (18%) developed BK viremia. At a median follow-up of 30 months [IQR 24-36 months], median eGFR was 61 ml/min [IQR 53-73 ml/min] and the median urine protein-creatinine ratio was 0.14 g/g [IQR 0.1-0.31 g/g]. Seven patients (16%) had developed de-novo DSA – 3 developed class I (MFI range 1000-2200), 5 developed class II (MFI range 2400-14500). Using the iBox, the predicted median allograft survival will be 95% and 90% at 5 and 10-years in the future.
*Conclusions: Several years after trials of transplantation of HCV-viremic kidneys into uninfected patients, recipients have very good allograft function and little evidence of adverse consequences of infection. Multicenter studies with large samples are needed to determine if HCV infection is associated with rare complications.
| “$$graphic_” |
To cite this abstract in AMA style:
Potluri VS, Naqvi F, Reese P, Shah M, Brown D, Prenner S, Loupy A, Woodards A, Abt P, Blumberg E, Trofe-Clark J, Segev D, Bloom R, Sawinski D, Durand C, Porrett P, Levine M, Goldberg D, Desai N. Longer-Term Outcomes from the THINKER and EXPANDER Trials of Transplantation Using HCV-Viremic Kidneys [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/longer-term-outcomes-from-the-thinker-and-expander-trials-of-transplantation-using-hcv-viremic-kidneys/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress