Long-Term Safety in Epstein-Barr Virus (EBV)-Seropositive Kidney-Only Transplant Recipients Treated with Belatacept (BELA) in Clinical Practice: Final Study Results from the ENLiST Registry
C. Larsen1, F. Vincenti2, T. Kou3, C. Shadur4, B. Bresnahan5, S. Jordan6, E. S. Woodle7, N. Goes8, J. Vella9, D. Wojciechowski10, M. Polinsky3, A. Gomez-Caminero3
1Emory University Transplant Center, Atlanta, GA, 2UCSF Transplant Center, San Francisco, CA, 3Bristol-Myers Squibb, Princeton, NJ, 4Iowa Kidney Physicians, Des Moines, IA, 5Medical College of Wisconsin, Milwaukee, WI, 6Cedars-Sinai Medical Center, Los Angeles, CA, 7University of Cincinnati, Cincinnati, OH, 8Kaiser Permanente, San Francisco, CA, 9Maine Nephrology Associates, PA, Portland, ME, 10University Hospital Kidney and Liver Transplant Clinic, Dallas, TX
Meeting: 2020 American Transplant Congress
Abstract number: C-209
Keywords: Epstein-Barr virus (EBV), Post-transplant lymphoproliferative disorder (PTLD), Safety
Session Information
Session Name: Poster Session C: PTLD/Malignancies: All Topics
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: BELA, a selective T-cell costimulation blocker, is approved for the prophylaxis of organ rejection in EBV-seropositive adult kidney transplant recipients. In phase 3 BENEFIT and BENEFIT-EXT, BELA was associated with improved survival and renal function but also with a risk of post-transplant lymphoproliferative disorder (PTLD) estimated at 0.1-0.25 per 100 person-years (p-y) in EBV-seropositive patients (pts). This study examined long-term safety in EBV-seropositive kidney transplant recipients treated with BELA in the post-approval setting.
*Methods: This US-based, prospective, voluntary, multi-center registry (ENLiST) included adult EBV-seropositive kidney-only transplant recipients treated de novo (< 14 days of transplantation) with BELA. Primary objectives were to estimate incidence rates of confirmed PTLD, CNS PTLD, and progressive multifocal encephalopathy (PML). Minimum follow-up was 2 y.
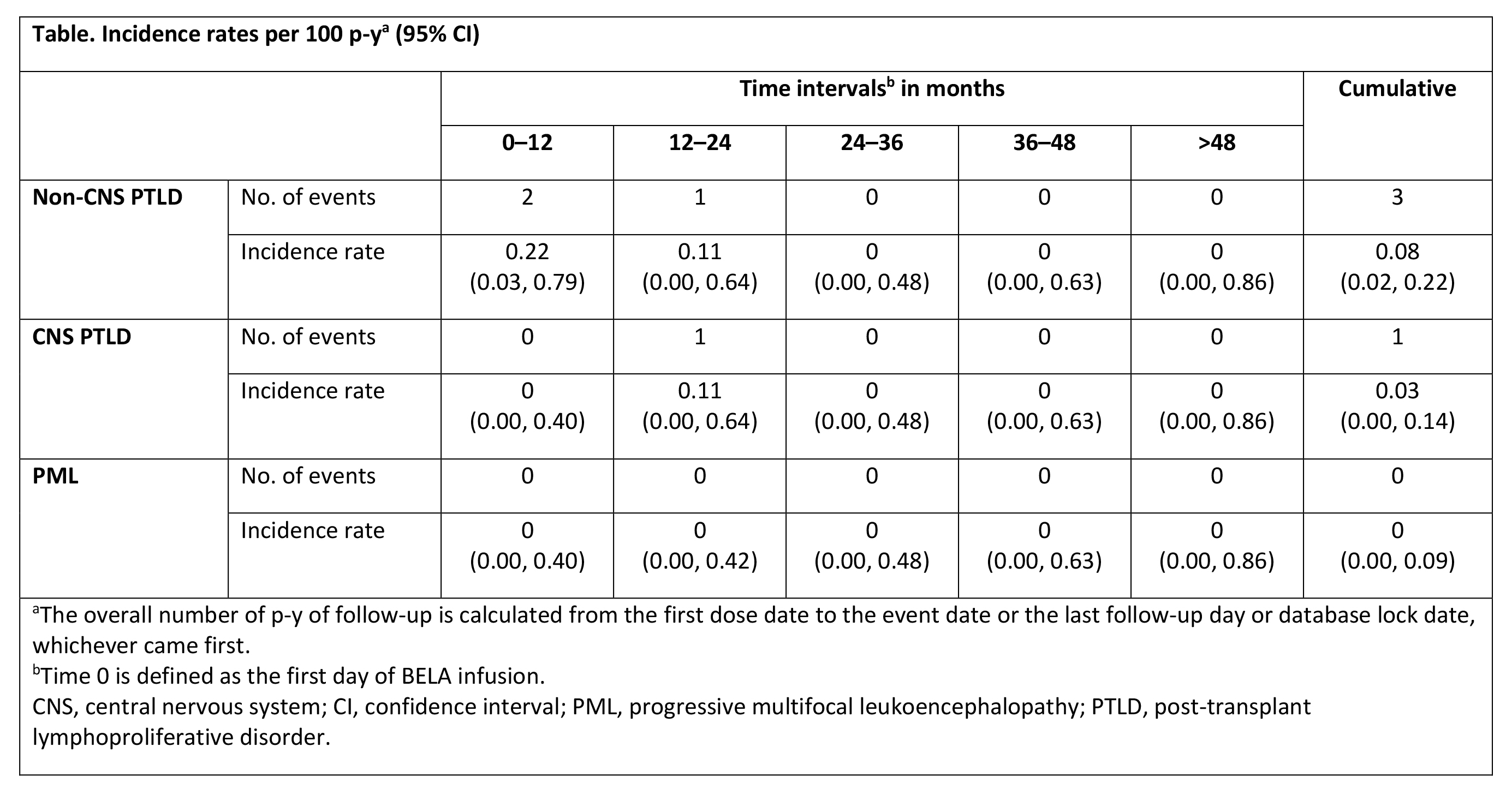
*Results: Between Feb 2012 and Apr 2017, 985 transplant recipients were enrolled; 933 EBV-seropositive pts were treated de novo with BELA. Mean age was 53 y, 63% were male, 54% were white, 65% were CMV seropositive, 95% were on CMV prophylaxis, and 56% received concomitant tacrolimus (TAC) and BELA at transplant; 84% of donors were EBV-seropositive. The mean duration of BELA exposure was 1314 ± 766 d. By study end, 3 cases of non-CNS PTLD, 1 case of CNS PTLD, and no cases of PML were reported. Two pts with non-CNS PTLD received concomitant TAC + BELA at transplant. The cumulative incidence rate was 0.08 per 100 p-y for non-CNS PTLD and 0.03 for CNS PTLD (Table). Incidence rates were comparable between pts with concomitant TAC + BELA at transplant and those without TAC (0.09 and 0.07 per 100 p-y, respectively; P = 0.9601). Of 4 pts with PTLD, 2 died due to PTLD and 2 were alive at study end.
*Conclusions: Incidence rates of PTLD and CNS PTLD among BELA-treated EBV-seropositive pts in the ENLiST registry were consistent with those observed in EBV-seropositive pts in previous clinical trials.
To cite this abstract in AMA style:
Larsen C, Vincenti F, Kou T, Shadur C, Bresnahan B, Jordan S, Woodle ES, Goes N, Vella J, Wojciechowski D, Polinsky M, Gomez-Caminero A. Long-Term Safety in Epstein-Barr Virus (EBV)-Seropositive Kidney-Only Transplant Recipients Treated with Belatacept (BELA) in Clinical Practice: Final Study Results from the ENLiST Registry [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-safety-in-epstein-barr-virus-ebv-seropositive-kidney-only-transplant-recipients-treated-with-belatacept-bela-in-clinical-practice-final-study-results-from-the-enlist-registry/. Accessed February 18, 2026.« Back to 2020 American Transplant Congress