Lipid Profiles Over 2 Years Follow-Up in De Novo Kidney Transplant Recipients Randomized to Once-Daily MeltDose® Tacrolimus (Envarsus® XR) Vs. Twice-Daily Tacrolimus (Prograf®): Results from a Phase 3, Double-Blind, Double-Dummy, Multicenter Trial
St. Barnabas Medical Center, Livingston
Erie County Medical Center, Buffalo
California Institute of Renal Research, San Diego.
Meeting: 2015 American Transplant Congress
Abstract number: B73
Keywords: Immunosuppression, Triglycerides
Session Information
Session Name: Poster Session B: Clinical Science: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Sunday, May 3, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Cardiovascular disease (CVD) is a leading cause of death in kidney transplant recipients (KTR). Focusing on modifiable CVD risk factors is key to decreasing CVD-related mortality. CNIs are associated with dyslipidemia, with cyclosporine generally associated with greater dyslipidemic effects than tacrolimus (tac). Envarsus® XR is a novel, extended-release, once-daily, MeltDose® formulation of tac which requires a lower total daily dose (TDD) vs. twice-daily tac (Prograf®) to achieve therapeutic target trough levels with lower Cmax and equivalent efficacy as Prograf. This analysis examined if differences in lipid profiles exist between Envarsus XR and Prograf. In this phase 3, double-blind, double-dummy, multicenter trial, de novo KTR were randomized to once-daily Envarsus XR (n=268) or twice-daily Prograf (n=275). Starting doses of Envarsus XR were 0.17mg/kg vs. 0.10mg/kg for Prograf; subsequent doses of drugs were adjusted to maintain tac trough levels within 6–11ng/mL for the first 30 days and 4–11ng/mL thereafter. TDD was lower from Month 1 onward for Envarsus XR and the difference between the 2 groups increased over time. At Month 24, the mean TDD for Envarsus XR was 24.4% lower than Prograf. LDL, HDL, and total cholesterol (TC) levels increased similarly in both groups from baseline; however mean levels generally stayed within recommended ranges. While both groups had an initial increase in triglycerides (TG) followed by a levelling, the mean percent change in TG levels from baseline to 24 months was significantly (p=0.049) less for Envarsus XR (82.4%+112.89) vs. Prograf (97.0%+106.38). These results show that both tac formulations are associated with a similar impact on HDL, LDL, and TC, with mean levels within recommended ranges. Mean percent increase in TG levels was significantly greater for Prograf compared to Envarsus XR with mean levels at 24 months higher than the recommended ≤150 mg/dL.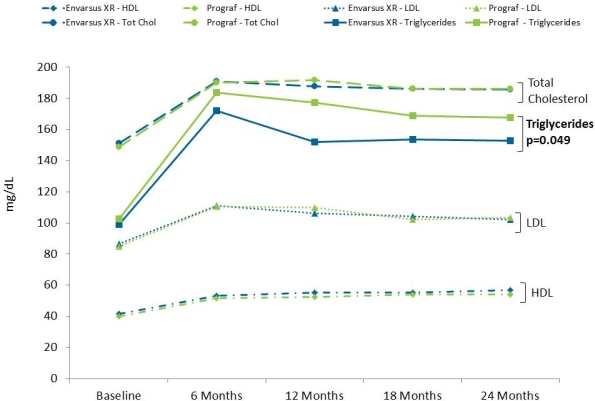
To cite this abstract in AMA style:
Mulgaonkar S, Pankewycz O, Steinberg S. Lipid Profiles Over 2 Years Follow-Up in De Novo Kidney Transplant Recipients Randomized to Once-Daily MeltDose® Tacrolimus (Envarsus® XR) Vs. Twice-Daily Tacrolimus (Prograf®): Results from a Phase 3, Double-Blind, Double-Dummy, Multicenter Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/lipid-profiles-over-2-years-follow-up-in-de-novo-kidney-transplant-recipients-randomized-to-once-daily-meltdose-tacrolimus-envarsus-xr-vs-twice-daily-tacrolimus-prograf-results-fr/. Accessed July 1, 2025.« Back to 2015 American Transplant Congress
