Ligation Of Hla Class Il Molecules Disrupts Endothelial Barrier Through Src-dependent Ve-cadherin Destabilization
1Department of Pathology and Laboratory Medicine, University of California, Los Angeles, Los Angeles, CA, 2Digestive Diseases Research Center, University of California, Los Angeles, Los Angeles, CA
Meeting: 2019 American Transplant Congress
Abstract number: 73
Keywords: Adhesion molecules, Alloantibodies, Endothelial activation, Rejection
Session Information
Session Name: Concurrent Session: Endothelial Cell Biology
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:30pm-3:42pm
Presentation Time: 3:30pm-3:42pm
Location: Room 310
*Purpose: Microvascular inflammation, vascular permeability, and accumulation of intravascular mononuclear cells are characteristics of acute and chronic antibody-mediated rejection. Vascular endothelial cadherin (VE-Cadherin) is a major architectural protein that regulates the endothelial barrier by controlling endothelial cell (EC) permeability and subsequent transendothelial migration of immune subsets. Despite being a significant clinical concern for allograft and patient survival, the mechanisms underlying how antibodies against HLA class II (HLA II Ab) activate the endothelium and mediate inflammation remain poorly understood.
*Methods: Recombinant Class II Transactivator (CIITA) was sub-cloned with pAd/PL-DEST vector to achieve expression of HLA II on vascular endothelium, and the effects of HLA II Ab on EC permeability including VE-Cadherin phosphorylation, internalization and signaling were determined using immunoblotting and multicolor immunofluorescence confocal microscopy.
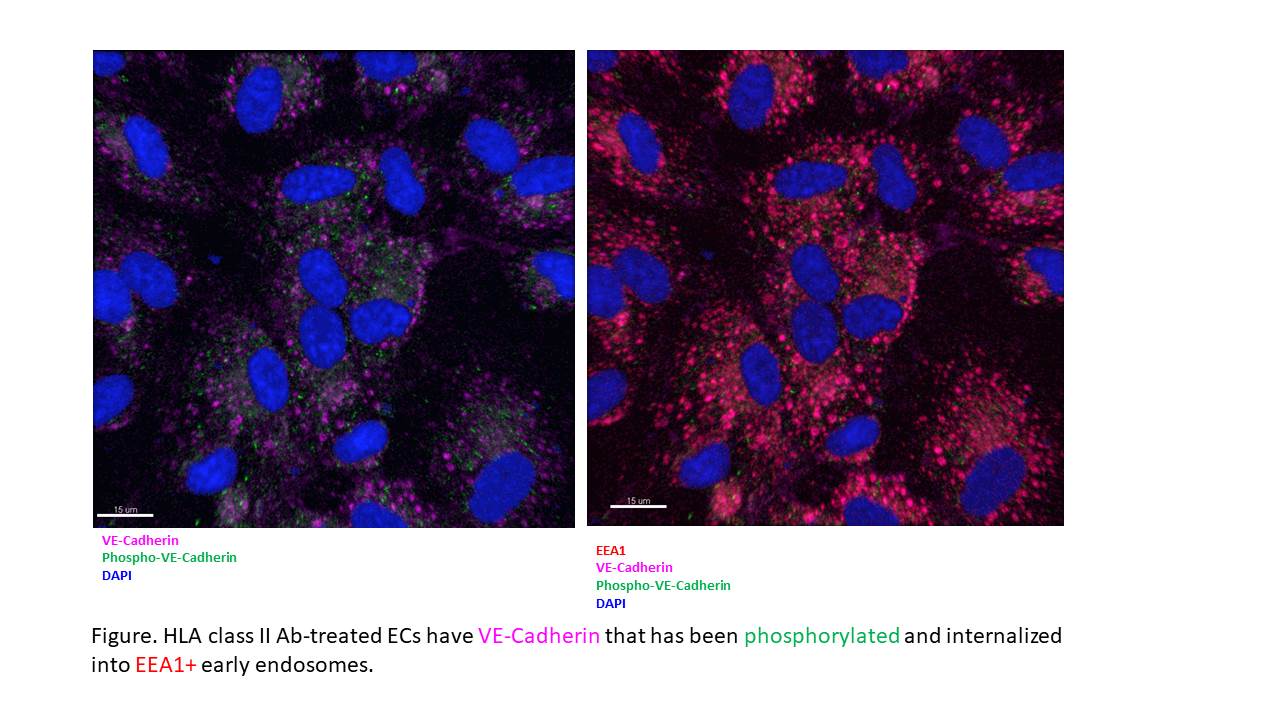
*Results: Three-dimensional subcellular reconstruction of multiplex confocal images revealed HLA II Ab increased VE-Cadherin phosphorylation at Tyr685 and internalization into EEA1+ early endosomes (Fig), thereby disassembling intercellular junctions and contributing to EC permeability. HLA II Ab activated both Src and ERK; however, HLA II-Ab-induced VE-Cadherin internalization was dependent only on Src, as both pharmacological and siRNA inhibition of Src, but not of ERK, attenuated HLA II Ab-induced phosphorylation of VE-Cadherin at Tyr685 as well as its internalization into EEA1+ early endosomes.
*Conclusions: HLA II Ab induces rapid endocytosis of VE-Cadherin via a Src-dependent tyrosine phosphorylation of VE-Cadherin, thereby disrupting the endothelial barrier function and contributing to vascular permeability, providing a potential therapeutic target for prevention of transplant antibody-mediated rejection.
To cite this abstract in AMA style:
Li F, Sosa RA, Valenzuela NM, Rozengurt E, Reed EF. Ligation Of Hla Class Il Molecules Disrupts Endothelial Barrier Through Src-dependent Ve-cadherin Destabilization [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/ligation-of-hla-class-il-molecules-disrupts-endothelial-barrier-through-src-dependent-ve-cadherin-destabilization/. Accessed February 25, 2026.« Back to 2019 American Transplant Congress