Kidney Transplant Results Comparing Two Formulations of Prolonged-Release Tacrolimus: Paired Study of Kidney Donors
Nephrology Department, Regional University Hospital, University of Malaga, IBIMA, REDinREN (RD16/0009/0006), Málaga, Spain
Meeting: 2020 American Transplant Congress
Abstract number: A-086
Keywords: Calcineurin, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Although the formulations of prolonged-release tacrolimus (Advagraf® and Envarsus®) are effective after kidney transplantation in terms of efficacy and safety, no paired analysis has been done comparing the two formulations with transplants from the same donor. The objetic of this study was to analyze the results after deceased donor kidney transplantation comparing two formulations of prolonged-release tacrolimus (Advagraf® versus Envarsus®).
*Methods: We performed a prospective study with a paired analysis of kidney donors using Advagraf® for the first transplant undertaken from the same donor if the date was even and Envarsus® if it was odd. Data were collected during one year post-transplant on graft and patient survival, kidney function, tacrolimus levels and dose and coefficient of variation, acute rejection, infections and post-transplant diabetes.
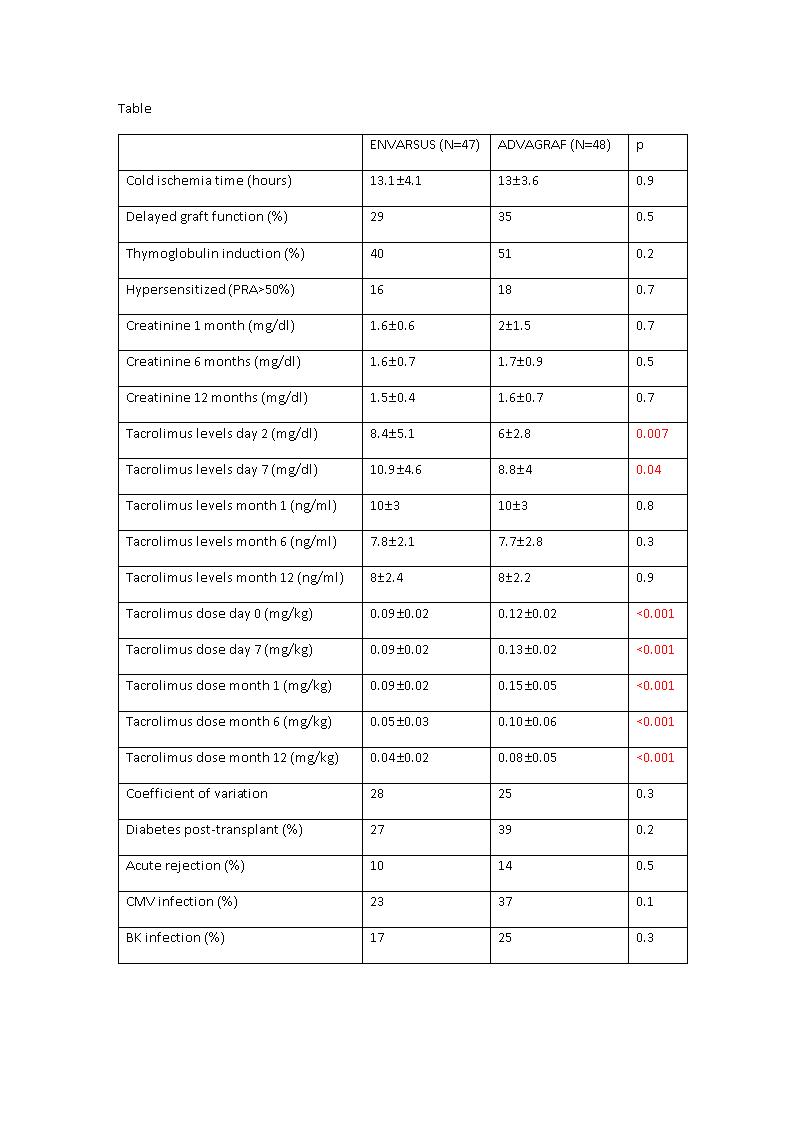
*Results: The study included 108 kidney transplants; 54 patients received treatment with Advagraf® and 54 with Envarsus®. The mean age of the donors was 57±11 years and 63% were male; 40% had hypertension and 8% diabetes. No differences were found between the groups in the recipient characteristics. In 13 patients it was necessary to change the formulation to Prograf® (7 in the Envarsus group and 6 in the Advagraf group). We found no differences between the Advagraf and Envarsus groups in any of the results, except for the tacrolimus levels during the first week and the dose needed during the follow-up (Table). The percentage of acute rejections was 14% in the Advagraf group versus 10% in the Envarsus group (p=0.5). Censored graft survival was significantly greater in the Envarsus group (100% for Envarsus® versus 91% for Advagraf®; p=0.04) and patient survival was 100% versus 95% (p=0.1).
*Conclusions: Envarsus® enables higher tacrolimus levels to be achieved during the first post-transplant week despite receiving lower doses. This could explain the tendency to a greater immune dysfunction in the Advagraf group and the better graft survival. Other parameters of safety and efficacy were similar between the two formulations.
To cite this abstract in AMA style:
Jimenez VLopez, Titos JAlonso, España JGamez, Díaz MCabello, Marrero DHernández. Kidney Transplant Results Comparing Two Formulations of Prolonged-Release Tacrolimus: Paired Study of Kidney Donors [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/kidney-transplant-results-comparing-two-formulations-of-prolonged-release-tacrolimus-paired-study-of-kidney-donors/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress