Increase in 48 Month Cumulative Acute Rejection Rates in the Most Recent Cohort: The 2018 Transplant Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS)
1Washington University in St. Louis, Saint Louis, MO, 2Mayo Clinic, Rochester, MN, 3Duke University, Durham, NC, 4Children's National Medical Center, Washington, DC, 5Emmes Corporation, Rockville, MD, 6BC Children's Hospital, Vancouver, BC, 7Seattle Children's Hospital, Seattle, WA, 8Johns Hopkins University, Baltimore, MD
Meeting: 2019 American Transplant Congress
Abstract number: 344
Keywords: Kidney transplantation, Multicenter studies, Pediatric, Rejection
Session Information
Session Name: Concurrent Session: Kidney: Pediatrics II
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 304
*Purpose: The NAPRTCS registry has collected data on children receiving kidney transplants in North America since 1987. The registry has served to document and influence practice patterns, clinical outcomes, and changing trends.
*Methods: Data are submitted voluntarily by centers on patients transplanted before their 21st birthday, at the time of transplant, one and six months post-transplant, and every six months thereafter. Collected data include peri-transplant information, donor and recipient characteristics, immunosuppression, acute rejection (AR), malignancy, height, graft and patient survival. Chronic antibody-mediated rejection has not been tracked. This analysis reports on the outcomes up to the end of December 2017.
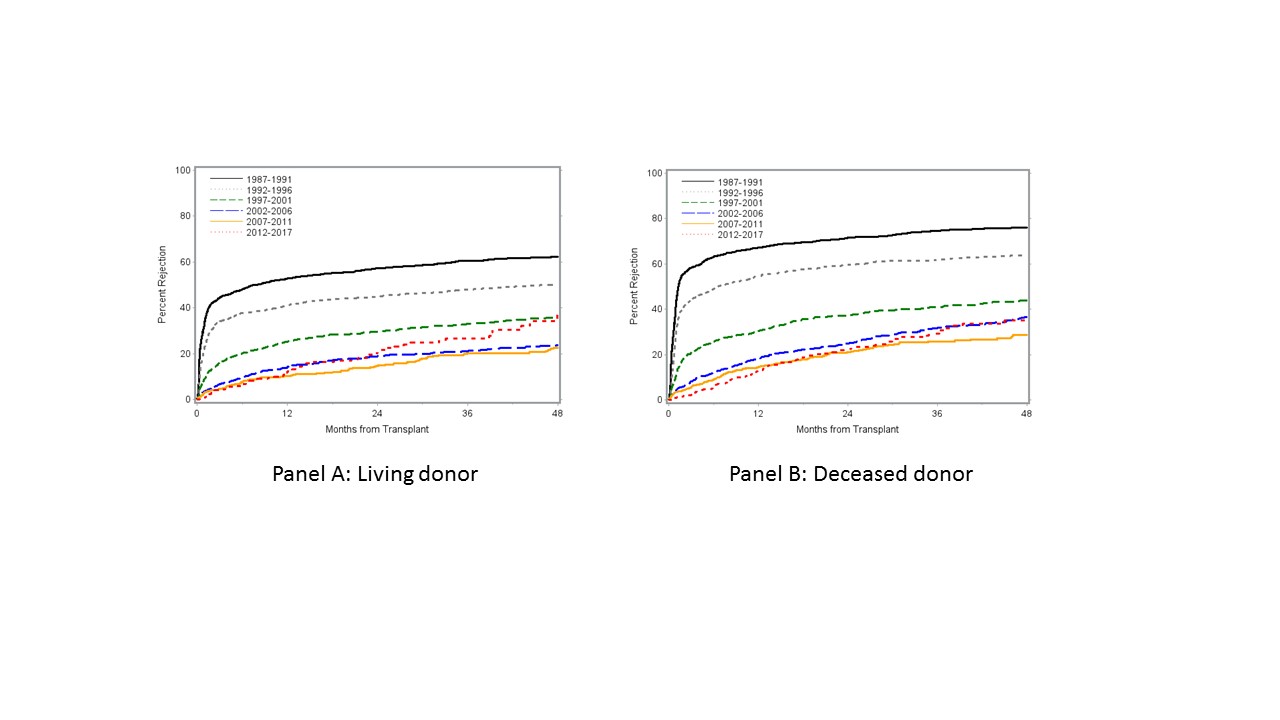
*Results: In 12,920 renal transplants into 11,870 children, 10,032 were primary, 2437 of those being pre-emptive. Recipients remain 60% male and age distribution remains stable (0-1: 5.5%, 2-5: 15.3%, 6-12: 32.3%, 13-17: 38.8%) but white recipients are now below 50%. In the most recent 2008-2017 period, a tacrolimus-mycophenolate-prednisone or tacrolimus-mycophenolate combination was used in > 80% of patients. While the 12-month AR rate is now very low at 12-13%, the cumulative 48 month AR rate of nearly 40% is now higher in the most recent cohort than in the prior two cohorts for living donor transplants, and to the most recent cohort for deceased donor transplants (Figure). Kaplan-Meier univariable analyses did not show higher late first AR rates in steroid-free or higher HLA mismatch patients. Several risk factors that significantly increased or decreased AR risk for both living and deceased donor transplants in older eras, such as use of induction therapy, recipient black race or more recent transplant year, are now no longer significant.
*Conclusions: While rates of early AR have steadily improved, the rise in cumulative 48 month AR rates warrants concern.
To cite this abstract in AMA style:
Dharnidharka V, Cramer C, Chua A, Moudgil A, Martz K, Blydt-Hansen T, Smith J, Neu A. Increase in 48 Month Cumulative Acute Rejection Rates in the Most Recent Cohort: The 2018 Transplant Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/increase-in-48-month-cumulative-acute-rejection-rates-in-the-most-recent-cohort-the-2018-transplant-report-of-the-north-american-pediatric-renal-trials-and-collaborative-studies-naprtcs/. Accessed February 23, 2026.« Back to 2019 American Transplant Congress