Incidence, Timing, and Consequence of De Novo Donor Specific Antibody in Intestinal Transplantation
Center for Intestinal Care and Transplant, Medstar Georgetown Transplant Institute, Washington, DC.
Meeting: 2015 American Transplant Congress
Abstract number: 414
Keywords: Antibodies, Intestinal transplantation
Session Information
Session Name: Concurrent Session: Small Bowel Transplantation
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:39pm-2:51pm
Presentation Time: 2:39pm-2:51pm
Location: Room 117
The development of denovo Donor specific antibody (dnDSA) in intestinal transplantation (ITx) remains largely unknown. We conducted a prospective and longitudinal analysis of adult and pediatric ITx recipients to determine the incident, timing, and consequence of dnDSA in ITx.
A prospective DSA monitoring program was instituted in June 2013. Monitoring included weekly DSA analysis for 2 months, then monthly testing for the remaining year. If a dnDSA appears, then weekly monitoring is reinstituted.
25 ITx were performed from June2013 to October 2014. 13 adult and 12 pediatric cases were performed with one graft loss. All recipients are alive. Mean followup time is 276 ± 161 days. 16 were isolated intestine, 4 liver/intestine, 4 multivisceral, and 1 modified multivisceral. 10 recipients were sensitized (median PRA= 56%, range 2-94%), 6 highly sensitized (PRA >20%) (median PRA = 61.5%, range 30-94%). At the time of transplant, T cell and B cell flowcytometric crossmatching were negative in all recipients. dnDSA developed in 8/25 (32%) recipients (3 peds/5 adult) at a median time after transplant of 22.5 days (range 6-33 days). Overall 1 year freedom from dnDSA was 68% (Figure 1).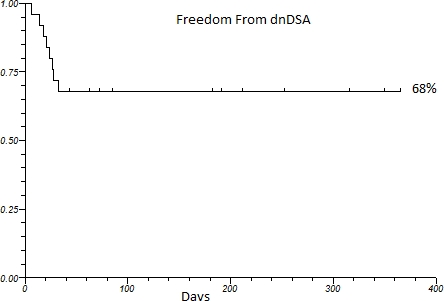 No difference in the incidence of dnDSA with regards to sensitized (p =0.85) or highly sensitized (p=0.52) recipients versus unsensitized recipients. Liver inclusion was not associated with any difference in the development of dnDSA (p =0. 21). Histological rejection was observed in 4 recipients, 3 (75%) in conjunction with the development of dnDSA.
No difference in the incidence of dnDSA with regards to sensitized (p =0.85) or highly sensitized (p=0.52) recipients versus unsensitized recipients. Liver inclusion was not associated with any difference in the development of dnDSA (p =0. 21). Histological rejection was observed in 4 recipients, 3 (75%) in conjunction with the development of dnDSA.
The development of dnDSA occurs at a rapid and high incidence after ITx. Aggressive monitoring and mitigation of dnDSA may contribute towards improved long term outcome in ITx.
To cite this abstract in AMA style:
Matsumoto C, Hawksworth J, Kozlowski S, Rosen-Bronsen S, Kromer A, Grafals M, Yazgi N, Kaufman S, Khan K, Girlanda R, Island E, Desai C, Fishbein T. Incidence, Timing, and Consequence of De Novo Donor Specific Antibody in Intestinal Transplantation [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/incidence-timing-and-consequence-of-de-novo-donor-specific-antibody-in-intestinal-transplantation/. Accessed March 8, 2026.« Back to 2015 American Transplant Congress
