Improved Medication Safety Outcomes with a Mobile Health, Pharmacist-Led Intervention: Results of the TRANSAFE Rx Randomized Controlled Trial
MUSC, Charleston, SC
Meeting: 2020 American Transplant Congress
Abstract number: 434
Keywords: Kidney transplantation, Methodology
Session Information
Session Time: 9:59am-10:40am
 Presentation Time: 10:10am-10:17am
Presentation Time: 10:10am-10:17am
Location: Main Channel
*Purpose: Medication (med) safety events are a predominant cause of poor health outcomes, particularly within high-risk groups such as kidney transplant (KTX) recipients. The primary aim of the TRANSAFE Rx study was to assess the efficacy of a pharmacist-led, mHealth-based intervention at reducing med errors, as compared to usual care, in KTX.
*Methods: This was a 12-month, parallel, two-arm, 1:1 randomized controlled clinical trial in adult KTX 6 to 36 months post-transplant (NCT03247322). All received usual post-transplant care, while those randomized to the intervention arm received clinical pharmacist-led supplemental med therapy monitoring and management via a smartphone-enabled mHealth application, bioinformatic web portal, risk-based televisits, and home-based BP and glucose monitoring. Med errors were assessed by blinded study coordinators and pharmacists every 2 months throughout the study in each arm. Negative binomial random intercept generalized linear mixed model regression was used to determine the impact of the intervention on primary outcome of med error rate.
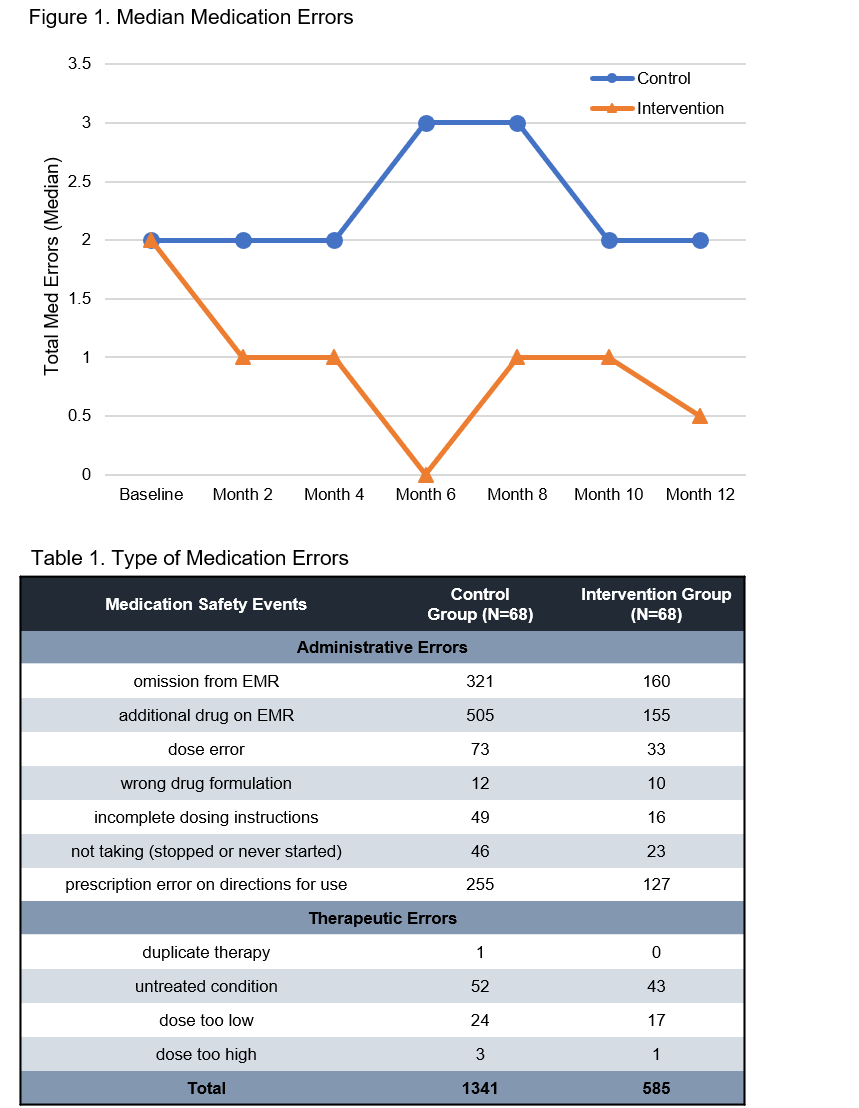
*Results: 136 patients were enrolled (68 in each arm), 2 withdrew before completing for a 98.5% retention rate. Baseline characteristics were similar between arms, except for more DM in the control arm at randomization. The intervention produced a 64% reduction in the total number of med errors (585 vs. 1341, p<0.001). Adjusted modeling demonstrated a 10% reduction in med error rates per month (p<0.001), with an overall 12-month reduction of two thirds (IRR 0.33, 95% CI 0.23 to 0.47; p<0.0001). Common errors included omissions, additions and wrong dose, with undertreated conditions being a common therapeutic error (Table 1). During the 12-month study there were no acute rejections or graft losses in the intervention arm, while there were 3 rejections (4.4%) and 4 graft losses (5.9%) in the control arm.
*Conclusions: A pharmacist-led mHealth intervention significantly improved medication safety in KTX recipients. This intervention represents a promising mechanism to improve medication safety and clinical outcomes in this high-risk patient population.
To cite this abstract in AMA style:
Gonzales H, Fleming J, Gebregziabher M, McGillicuddy J, Taber D. Improved Medication Safety Outcomes with a Mobile Health, Pharmacist-Led Intervention: Results of the TRANSAFE Rx Randomized Controlled Trial [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/improved-medication-safety-outcomes-with-a-mobile-health-pharmacist-led-intervention-results-of-the-transafe-rx-randomized-controlled-trial/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress