Implementation of Pre-Transplant Infectious Disease Risk Assessment: Sustained Success With Rapid-Cycle Improvement
Cincinnati Children's Hospital Medical Center, Cincinnati, OH.
Meeting: 2015 American Transplant Congress
Abstract number: C80
Keywords: Infection, Pediatric, Risk factors
Session Information
Session Name: Poster Session C: Infections Risks/Prevention and Immunosuppression
Session Type: Poster Session
Date: Monday, May 4, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Pre-transplant infectious disease risk assessment can identify and mitigate potential risk for transplant candidates to improve post-transplant outcomes. We aimed to improve pre-transplant ID risk assessment in pediatric solid organ transplant candidates (n=74) within a tertiary care pediatric hospital through a multidisciplinary coordinated quality improvement effort that was not dependent on ID consultation. Screening for three targeted pathogens (TB, MRSA and Hepatitis A) and immunization plan initiation comprised the composite goal. Targeted pathogens were chosen to address baseline gaps and to approach different screening processes (serology, skin testing, culture). Immunizations required collection and review of verified immunization records followed by plan development and initiation. Immunization plan initiation represented the largest practice gap and most complex process in the bundle. Rapid cycle parallel interventions after evaluation of key drivers and process mapping focused on education, communication, standardization of order sets, and immunization planning. Composite goal attainment requiring all three screens plus immunization plan implementation improved from a baseline of 30% (n=34) to over 90% (n=40) in a 2 month period and has been 100% for 9 months except for an urgent transplant performed within 24 hours of presentation. Multidisciplinary coordination of quality improvement efforts can facilitate the integration of infectious disease risk assessment into transplant candidate evaluation regardless of ID consultation. 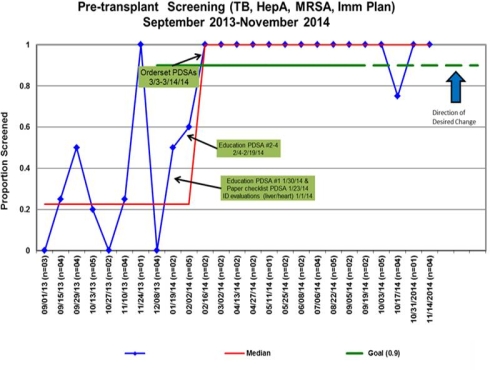
To cite this abstract in AMA style:
Danziger-Isakov L, Blum S, Dahale D, Morrow B, Rengering R, Schoborg D, Stark S, Wells E, Lorts A, Kotagal U, Bucuvalas J. Implementation of Pre-Transplant Infectious Disease Risk Assessment: Sustained Success With Rapid-Cycle Improvement [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/implementation-of-pre-transplant-infectious-disease-risk-assessment-sustained-success-with-rapid-cycle-improvement/. Accessed June 30, 2025.« Back to 2015 American Transplant Congress
