Implementation of Infectious Disease Surveillance in Recently Deceased Human Xenograft Study
NYU Langone Transplant Institute, New York, NY
Meeting: 2022 American Transplant Congress
Abstract number: 1525
Keywords: Infection, Kidney transplantation, Monitoring, Pig
Topic: Basic Science » Basic Science » 13 - Xenotransplantation
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
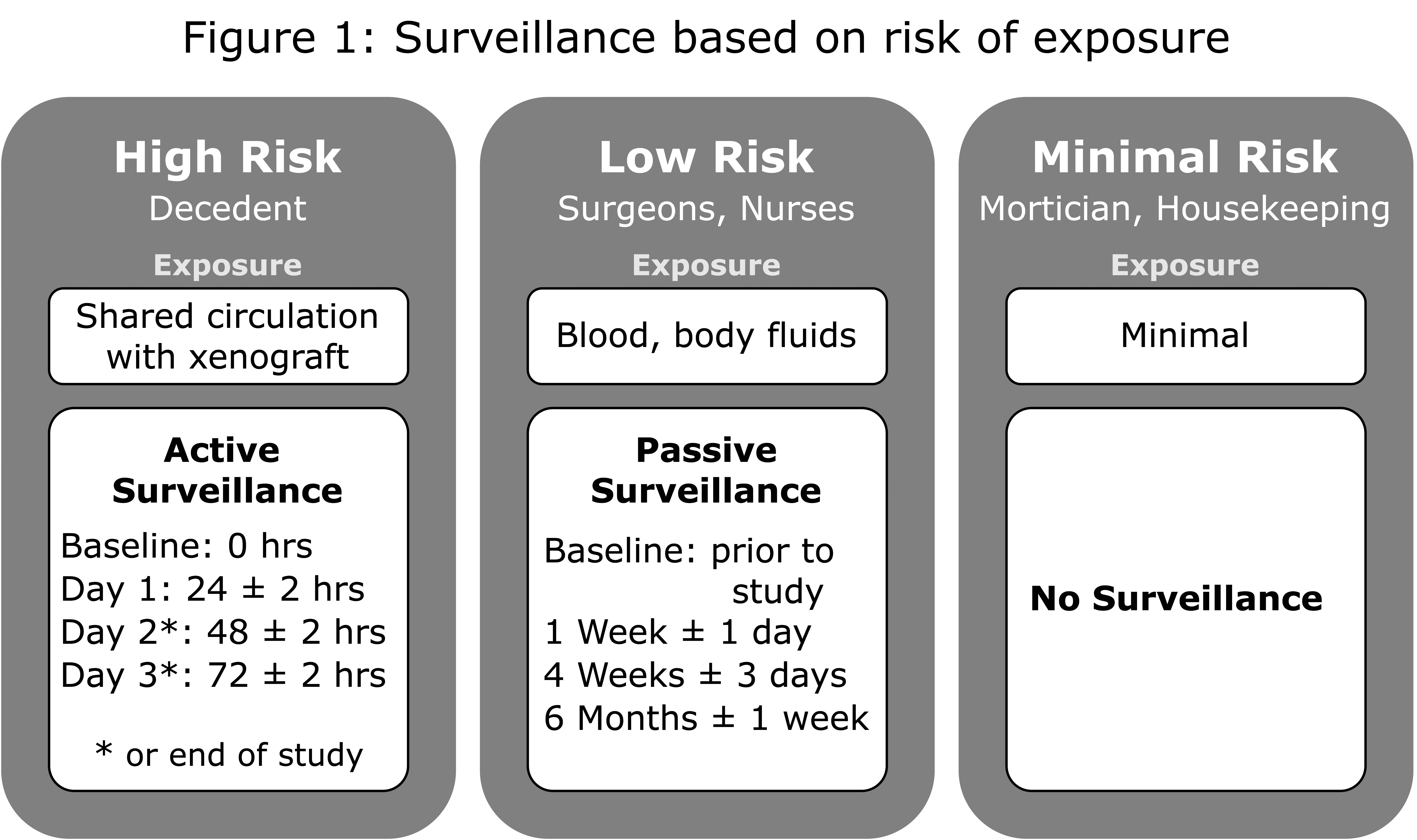
*Purpose: Xenotransplantation is a promising solution for the ongoing organ shortage but has been slow to develop because of both immunological challenges and potential risk of zoonosis. We developed and implemented an infectious disease surveillance protocol for transplantation of a genetically engineered porcine kidney into a brain-dead human subject (decedent) for a period of 2-3 days to assess renal function and early rejection.
*Methods: We reviewed the medical literature, national regulatory policies on human xenotransplantation, and donor pig herd specific infectious control protocols for zoonotic pathogen risk stratification and development of a testing and sample storage protocol. Decedent peripheral blood mononuclear cells (PBMCs) were tested for porcine endogenous retrovirus (PERV) A, B, and C mRNA production by reverse transcriptase-PCR (RT-PCR) and DNA integration by quantitative real-time PCR. Primers overlapped with all PERV subtypes (A, B, C) and PERV C specifically. Decedent PBMCs were compared to a positive control PERV C positive pig sample and were also tested for pRPL4, a pig housekeeping gene, to detect microchimerism from pig leukocytes potentially present in the organ.
*Results: We identified PERV as the primary pathogen with possible risk of transmission from porcine xenografts. Our risk mitigation protocol included standard infection control measures, active surveillance for PERV in the decedent, and passive storage of samples assessed to have potential risk of exposure to transmissible zoonotic pathogens from the decedent. Infection control included training study personnel in appropriate personal protective equipment use and on-site embalming of the decedent at study conclusion. We developed an active surveillance testing protocol for the decedent and a passive surveillance protocol for personnel, both including collection of plasma and frozen PBMCs, with storage for future testing if indicated (Figure 1). In the first xenotransplant utilizing this protocol, decedent PBMCs drawn at baseline, 6 hours, and 24 hours were negative for PERV A, B, and C and pRPL4 using RT-PCR and quantitative real-time PCR.
*Conclusions: In our first xenotransplant, there was no evidence of transmission of PERV or microchimerism in the human decedent. These findings support the low risk of transmission of PERV in humans receiving porcine kidneys. Future studies with increased time of exposure to xenograft would further evaluate the safety of xenotransplantation and strengthen the possibility of a renewable source of transplantable organs.
To cite this abstract in AMA style:
Cawthon SJ, Weldon EP, Lawson NS, Lonze BE, Montgomery RA, Mehta SA. Implementation of Infectious Disease Surveillance in Recently Deceased Human Xenograft Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/implementation-of-infectious-disease-surveillance-in-recently-deceased-human-xenograft-study/. Accessed February 19, 2026.« Back to 2022 American Transplant Congress