Impaired Humoral Response to Second Dose of BNT162b2 Messenger RNA COVID-19 Vaccine in Kidney Transplant Recipients in Japan: Prospective Observational Study
H. Sasaki1, K. Hasegawa1, M. Miura2, H. Harada1, D. Takamoto1, Y. Takada1, S. Harada1, H. Tanaka1
1Sapporo City General Hospital, Sapporo, Japan, 2Sapporo Hokuyu Hospital, Sapporo, Japan
Meeting: 2022 American Transplant Congress
Abstract number: 986
Keywords: Antibodies, COVID-19, Kidney transplantation, Vaccination
Topic: Clinical Science » Infection Disease » 25 - Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Information
Session Name: Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Hall C
*Purpose: The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has a major impact on solid organ transplant (SOT) recipients, with mortality rate up to 22%. The effect of SARS-CoV-2 vaccination are known to have poor responses even after 2nddose in SOT in Europe and the United States. We investigated immune response by detecting SARS-CoV-2 antibody (Ab) post two-doses of messenger RNA (mRNA)-based SARS-CoV-2 vaccinesin Japanese renal transplant recipients as prospective observational study.
*Methods: 352 recipients who have no history of COVID-19, were confirmed no SARS-CoV-2 antibody before vaccination and received 2nd dose of BNT162b2 mRNA vaccine are enrolled in this study.Antibody detection test was performed by using the Roche Elecsys® Anti-SARS-CoV-2 immunoassay after more than 4weeks following 2nddose of it. Negative for N-Ab (0.4U/ml>) and positive for S-Ab (0.8U/ml<) considered to be positive for SARS-CoV-2 Ab.As a healthy control, SARS-CoV-2 Ab was determined in 990 healthy volunteers (HV) as well as 98 kidney donors as control for chronic kidney disease (Donor).
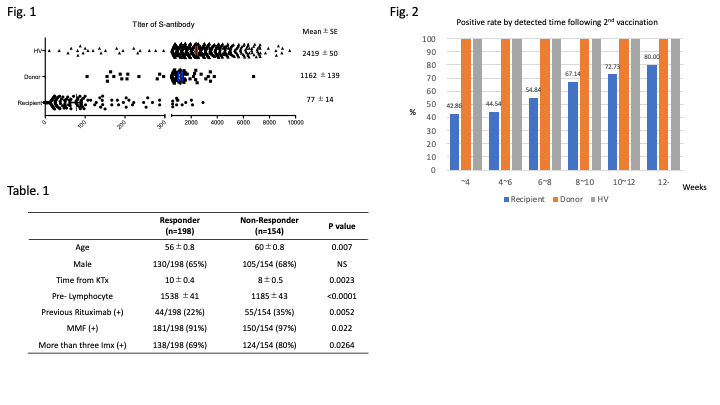
*Results: The rate of positive for S-Ab was 56.2% in recipients while that was 100% in HV and Donor. Titer of S-Ab (U/ml) was 77 in recipients although that was 2400 in HV and 1100 in Donor, respectively, which indicating significantly lower in recipients. (Fig. 1) Interestingly, positive rate for s-Ab by detecting time following 2ndvaccination in recipients was 44% in 4-6 weeks, 54% in 6-8 weeks, 67% in 8-10 weeks, 72% in 10-12 weeks, 80% in 12-weeks, which suggesting that recipients have delayed response to 2ndvaccination while that was 100% in any time point in HV and Donor. (Fig.2) Moreover, to elucidate difference between responder (n=198) and non-responder (n=154) in recipients, we compared clinical background that influencedoutcome. In non-responder, there were significant difference in older age at vaccination, less lymphocyte, previousdoses of rituximab, concomitant use of mycophenolate mofetil and oral administration of more than three immunosuppressants. (Table.1)
*Conclusions: Kidney recipients have delayed response with lower titer of SARS-CoV-2 antibodyfollowing second dose of mRNA-based COVID-19 Vaccine.
To cite this abstract in AMA style:
Sasaki H, Hasegawa K, Miura M, Harada H, Takamoto D, Takada Y, Harada S, Tanaka H. Impaired Humoral Response to Second Dose of BNT162b2 Messenger RNA COVID-19 Vaccine in Kidney Transplant Recipients in Japan: Prospective Observational Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impaired-humoral-response-to-second-dose-of-bnt162b2-messenger-rna-covid-19-vaccine-in-kidney-transplant-recipients-in-japan-prospective-observational-study/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress