Impact of Variation in Histological Assessments and Their Impact on Clinical Trial Enrollment and Endpoints – Preliminary Report of The Gen04 Study
1Transplant Center, Mayo Clinic, Rochester, MN, 2Laboratory Medicine and Pathology, Mayo Clinic, Scottsdale, AZ, 3Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, 4Laboratory Medicine and Pathology, Mayo Clinic, Jacksonville, FL, 5Pathology, University of Szegad, Szegad, Hungary
Meeting: 2020 American Transplant Congress
Abstract number: 527
Keywords: Biopsy, Kidney transplantation, Protocol biopsy
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes V
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Renal allograft histology is often used as inclusion criteria for clinical trials. This includes Gen04, which is an NIH sponsored multi-center clinical trial involving a 1 year protocol biopsy. Subjects whose biopsies were Banff scored by Pathologists as normal (i/cg=0, ci>=0) or inflamed (cg=0, i>0, ci>0) were included in the validation of prognostic clinical trials involving molecular testing.
*Methods: A total of 491 subjects were prospectively enrolled in Gen04 from 02/2012-02/2015 at 4 sites. Subjects were adult conventional renal allograft recipients who had a 1 year biopsy performed as standard of care. Banff scores were obtained locally and entered into the study database. In addition all clinical slides created for light microscopy were sent to a central lab (>4800 slides) and scanned (Aperio AT2). A group of blinded central pathologists used a web-based program to perform a detailed assessment of biopsy features (53 questions, incl Banff scores). If Local and 1st central Path did not agree, then a 2nd central Path review was performed.
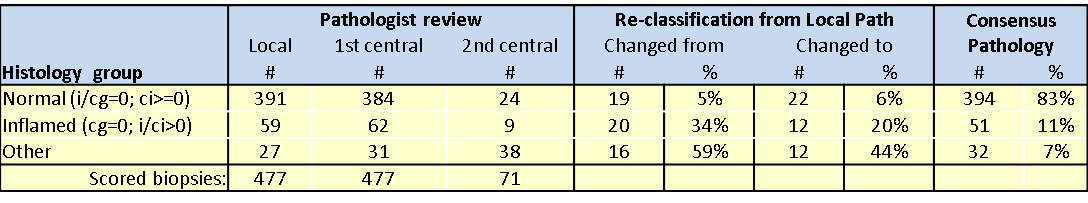
*Results: A total of 477 subjects had a 1 year surveillance biopsy scored locally with 391 cases as normal and 59 as inflamed (see Table). All cases underwent a 1st central pathology review and 71 cases needed a 2nd review to achieve consensus. Re-classification from local pathology was more common in the Inflamed group than the normal group. Nineteen (5%) cases changed from normal by Local path to non-normal by consensus. For inflamed histology, 20 cases (34%) changed from Inflamed Local path. Additionally, 22 cases (6%) scored by Local Path as non-normal were re-classified as normal by consensus and 12 cases (20%) scored by Local path as inflamed were re-classified as inflamed by consensus. After all Path reviews, 394 subjects (83%) had normal histology, 59 cases (11%) were inflamed and 32 cases (7%) had other histology.
*Conclusions: This study shows that as the complexity of histologic assessment increases (ie. inflammation), the more difficult it is to gain consensus among pathologists. Clinical trials that rely on pathologist interpretation for study enrollment or endpoints will benefit whole slide scanning (creates permanent record and easily shared) and central pathology assessment with multiple pathologists.
To cite this abstract in AMA style:
Park W, Ryan M, Hernandez LHerrera, Smith M, Geiger X, Sandor T, Cornell L, Stegall M. Impact of Variation in Histological Assessments and Their Impact on Clinical Trial Enrollment and Endpoints – Preliminary Report of The Gen04 Study [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-variation-in-histological-assessments-and-their-impact-on-clinical-trial-enrollment-and-endpoints-preliminary-report-of-the-gen04-study/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress