Impact of Respiratory Viral Panel Testing on Clinical Decision-Making Among Lung Transplant Recipients Based on Extent of Immunosuppression
1University of Maryland Medical Center, Baltimore, MD, 2University of Maryland School of Medicine, Baltimore, MD
Meeting: 2022 American Transplant Congress
Abstract number: 1500
Keywords: Immunosuppression, Lung infection, Lung transplantation, Polymerase chain reaction (PCR)
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Lung transplant recipients (LTRs) are at high risk for complications from viral respiratory infection, including chronic allograft dysfunction. Thus, multiplex molecular testing for respiratory pathogens, known colloquially as respiratory viral panel testing (RVP), has become a standard component of both surveillance and diagnostic bronchoscopy in LTRs. We sought to understand how RVP influences diagnosis and management among LTRs and whether the impact is increased among patients who are more severely immunosuppressed.
*Methods: All LTRs who underwent RVP testing at the University of Maryland Medical Center in Baltimore, MD between 2015 and 2020 were identified. For LTRs with multiple encounters during the study period, only the first encounter post-transplantation was considered eligible for inclusion. Impact of RVP on diagnosis and clinical management was determined via chart review. A random sample of charts was selected for this initial phase of the project. All RVP tests in this sample were obtained during symptomatic evaluation of LTRs in an acute care setting. Patients were considered more immunosuppressed if they were within one year of transplant or had a prior episode of acute rejection requiring treatment. Impact of RVP was compared between LTRs with and without severe immunosuppression by chi-square testing.
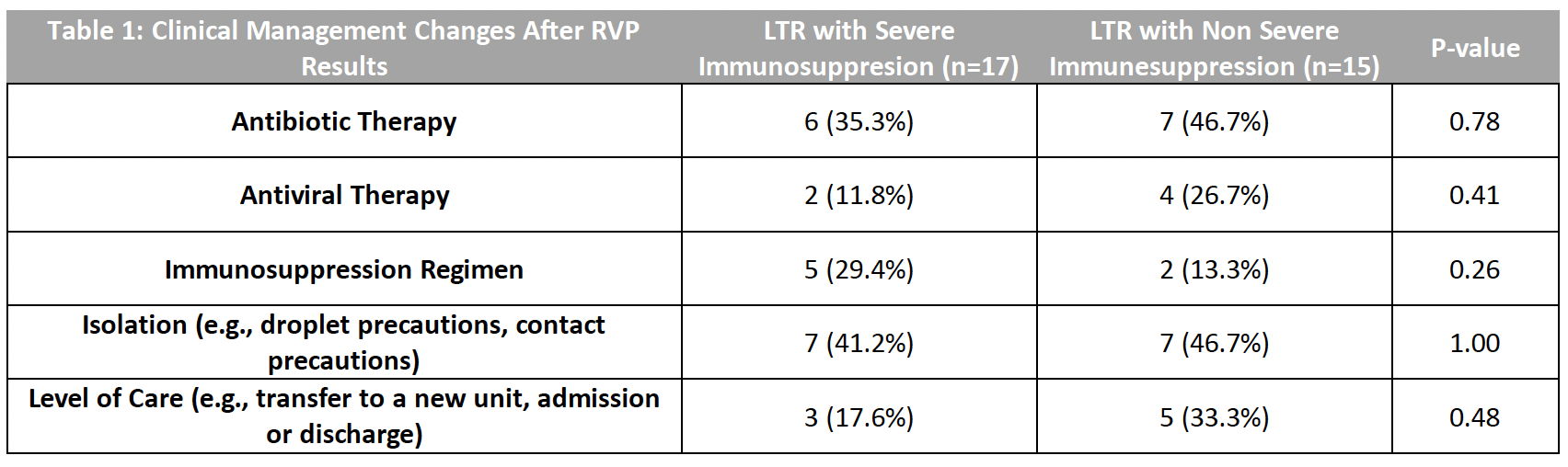
*Results: Out of 256 LTRs who underwent RVP testing during the study period, 32 (12.5%) charts were reviewed. On average, LTRs were 5.1 years after transplant at the time of RVP. In 21 cases (66% of 32), the admitting diagnosis was a respiratory complaint, and 9 RVPs were positive (28.1% of 32). The most common pathogen identified was rhinovirus (3 of 32, 9.4%). RVP was acknowledged as contributing to a diagnosis in 8 of 32 cases (25%), including 3 of 17 LTRs with severe immunosuppression and 5 of 15 without severe immunosuppression (p = 0.48). RVP facilitated clinical management in 11 LTRs (34% of 32), including 3 LTRs with severe immunosuppression and 8 LTRs without severe immunosuppression (p = 0.13). In 8 cases (25% of 32), RVP influenced clinical management by leading to a change in the level of care, which occurred in 3 of 17 (17.6%) with severe immunosuppression and 5 of 15 (33%) without severe immunosuppression (p = 0.48).
*Conclusions: RVP facilitates diagnosis or management in a substantial proportion of LTRs with respiratory illness. The impact of RVP does not appear to differ based on severity of immunosuppression.
To cite this abstract in AMA style:
Vostal AC, Lynen A, Chintalapati S, Uehling M, Prakash K, Shishido A, Baghdadi J. Impact of Respiratory Viral Panel Testing on Clinical Decision-Making Among Lung Transplant Recipients Based on Extent of Immunosuppression [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-respiratory-viral-panel-testing-on-clinical-decision-making-among-lung-transplant-recipients-based-on-extent-of-immunosuppression/. Accessed March 1, 2026.« Back to 2022 American Transplant Congress