Impact of Induction Immunosuppression Selection on Clinical Outcomes in Kidney Transplant Recipients During the COVID-19 Pandemic in New York City
Transplant Institute, NYU Langone Health, New York, NY
Meeting: 2021 American Transplant Congress
Abstract number: 889
Keywords: Immunosuppression, Induction therapy, Kidney, Rejection
Topic: Clinical Science » Kidney » Kidney: Acute Cellular Rejection
Session Information
Session Name: Kidney: Acute Cellular Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
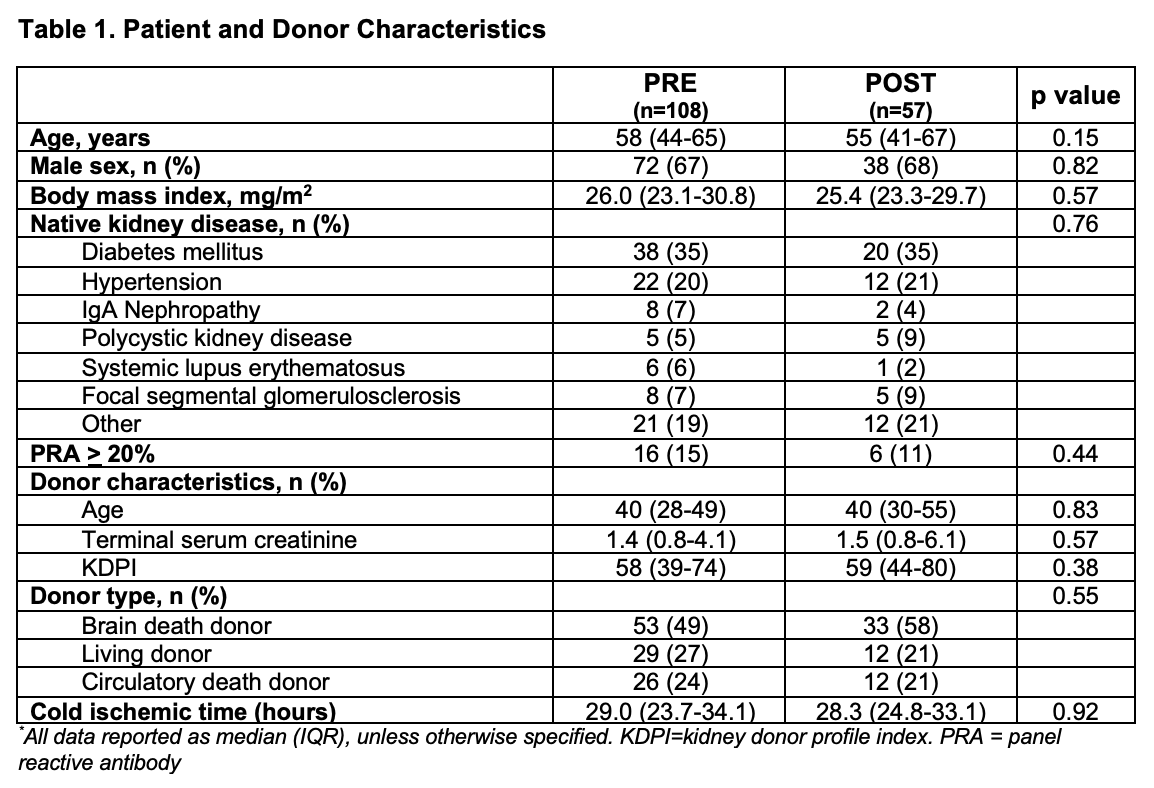
*Purpose: As an early epicenter in the coronavirus pandemic, our center modified induction immunosuppression strategies for transplantation. We sought to determine if changes in induction immunosuppression secondary to the COVID-19 pandemic impacted the incidence of acute rejection.
*Methods: Adult kidney transplant recipients at NYU Langone Health between 09/2019 and 08/2020 were retrospectively identified. Patients who received a multi-organ transplant or whose induction regimen was changed due to clinical course were excluded. Patients transplanted before and after 3/17/2020 were grouped as pre-pandemic (PRE) and post-pandemic (POST), respectively, based on temporary interruption of transplantation. Induction immunosuppression discordance was identified by blind adjudication from a standard protocol. Reduced induction agent use (basiliximab given when pre-pandemic protocol indicated rabbit anti-thymocyte globulin (rATG)) was compared between groups using a Chi-square test. Biopsy-proven acute rejection (BPAR) and the incidence of rejection was compared using a Poisson regression model.
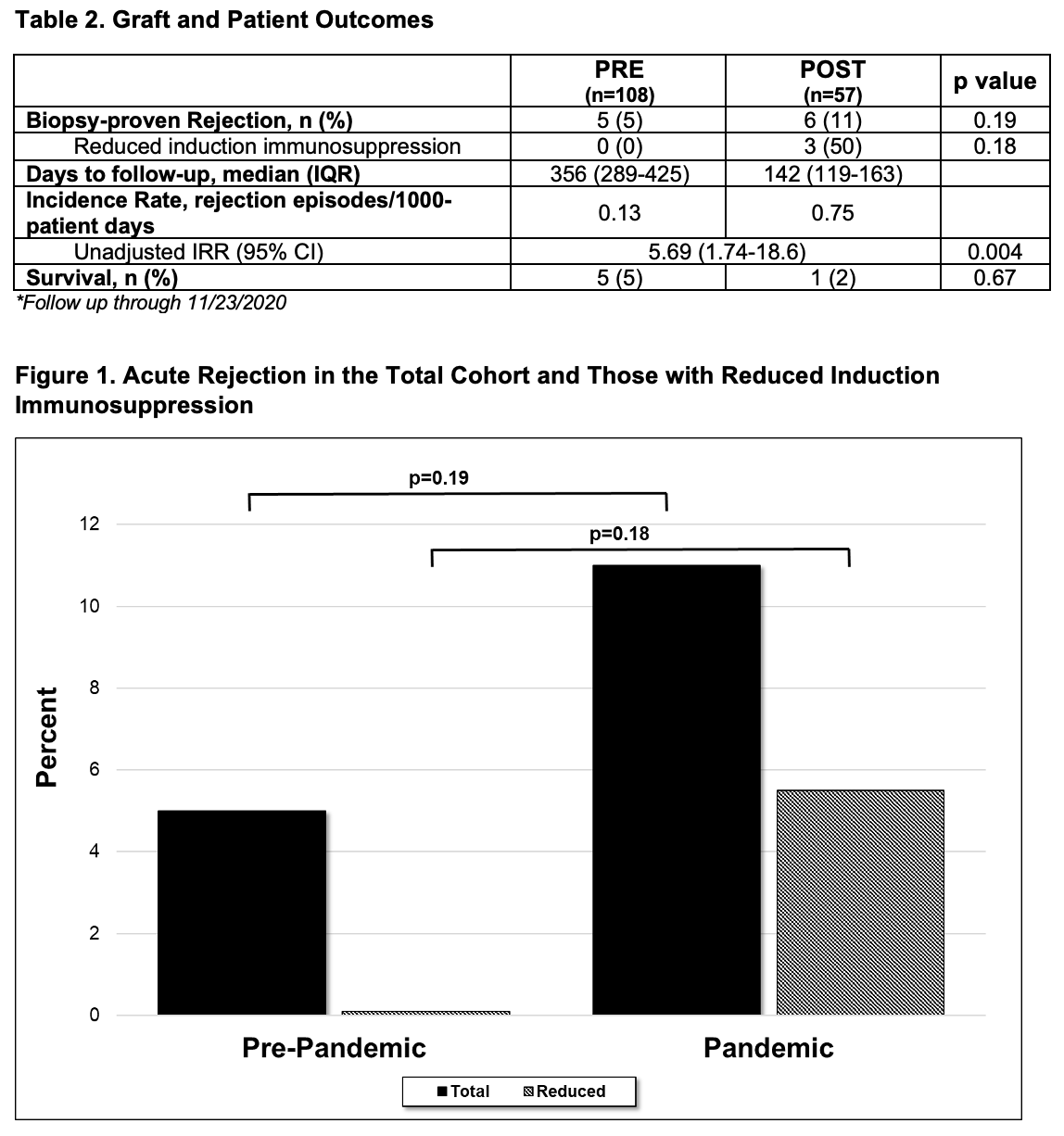
*Results: 203 kidney transplant recipients were retrospectively identified. 38 patients were excluded, leaving 165 patients for analysis. Median patient age was 57 years, 67% were male, and diabetes mellitus (35%) was the most common cause of renal disease. Discordance from protocol induction agent was 16% in the PRE group and 28% in the POST group (p=0.06). More patients received reduced induction with basiliximab in lieu of rATG in the POST group than the PRE group (26% vs. 7%, p=0.001). BPAR occurred in 5 PRE (5%) and 6 POST (11%) patients (p=0.19). The incidence of rejection was 0.13 and 0.75 rejection episodes/1,000-patient days for the PRE and POST groups, respectively; this was significantly different between the 2 time periods (unadjusted IRR 5.69, 95% CI 1.74-18.6, p=0.004).
*Conclusions: More patients received reduced induction immunosuppression driven by the COVID-19 pandemic concerns. These COVID-related changes in immunosuppression may have contributed to a trend in increased acute rejection in a preliminary analysis.
To cite this abstract in AMA style:
Khalil K, Jonchhe S, Stern J, Lewis TC, Alnazari N, Lonze B, Lewis ZStewart, Ali N. Impact of Induction Immunosuppression Selection on Clinical Outcomes in Kidney Transplant Recipients During the COVID-19 Pandemic in New York City [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-induction-immunosuppression-selection-on-clinical-outcomes-in-kidney-transplant-recipients-during-the-covid-19-pandemic-in-new-york-city/. Accessed February 17, 2026.« Back to 2021 American Transplant Congress