Impact of Donor Genotype on Recipient Tacrolimus Pharmacokinetics: The Liver Matters.
1U of Cinicnnati, Cincinnati
2Cincinnati Children's, Cincinnati
3iC42 Clinical Research, Colorado
Meeting: 2017 American Transplant Congress
Abstract number: D205
Keywords: Genomics, Immunosuppression, Pharmacokinetics, Risk factors
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Tacrolimus (Tac) is a cornerstone immunosuppressant metabolized in the gut and liver, resulting in high intra/inter patient pharmacokinetic (PK) variability. Recipient genotype (RG) impacts tac PK. CPIC guidelines recommend genotyping recipients to tailor tac dosing. Few studies have evaluated the impact of donor genotype (DG) following liver and small bowel transplantation, where DG and RG may differ.
Methods: 24 liver recipients (LT) and 18 kidney recipients (KT) in a prospective tac PK study had donor tissue genotyped for CYP3A5, CYP3A4 (*1B and *22), ABCB1, POR*28 following consent and authorization . Tac and metabolite PK parameters were obtained from 12-hour sampling analyzed via LC-MS/MS and derived using NONMEM. One-way ANOVA was used to evaluate genotype effect on apparent clearance (CL/F) and the area under the curve (AUC) ratios of tac to its main metabolite 13-O-desmethyltacrolimus (13-ODMT).
Results: PK parameters were well characterized; results for CYP3A5 variants are presented. Tac CL/F was not affected by DG in KT but recipient *1 variants significantly increased CL/F. Conversely, liver donor *1 variant had a higher CL/F whereas recipient did not (Fig 1). For 13-ODMT, kidney donor CYP3A5*1 had no impact on the 13-ODMT to tac AUC ratio whereas liver donor *1 increased it (Fig 2).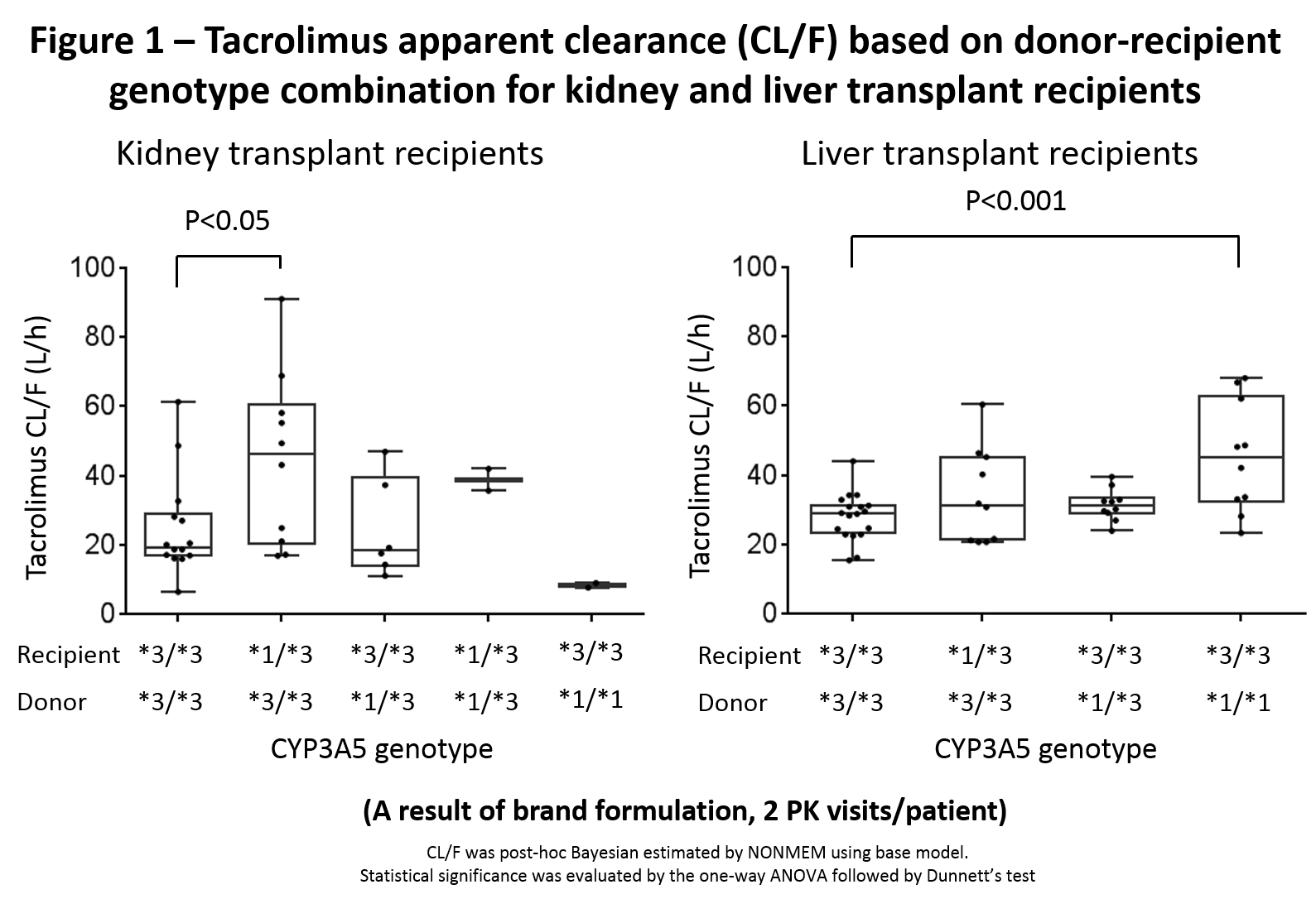
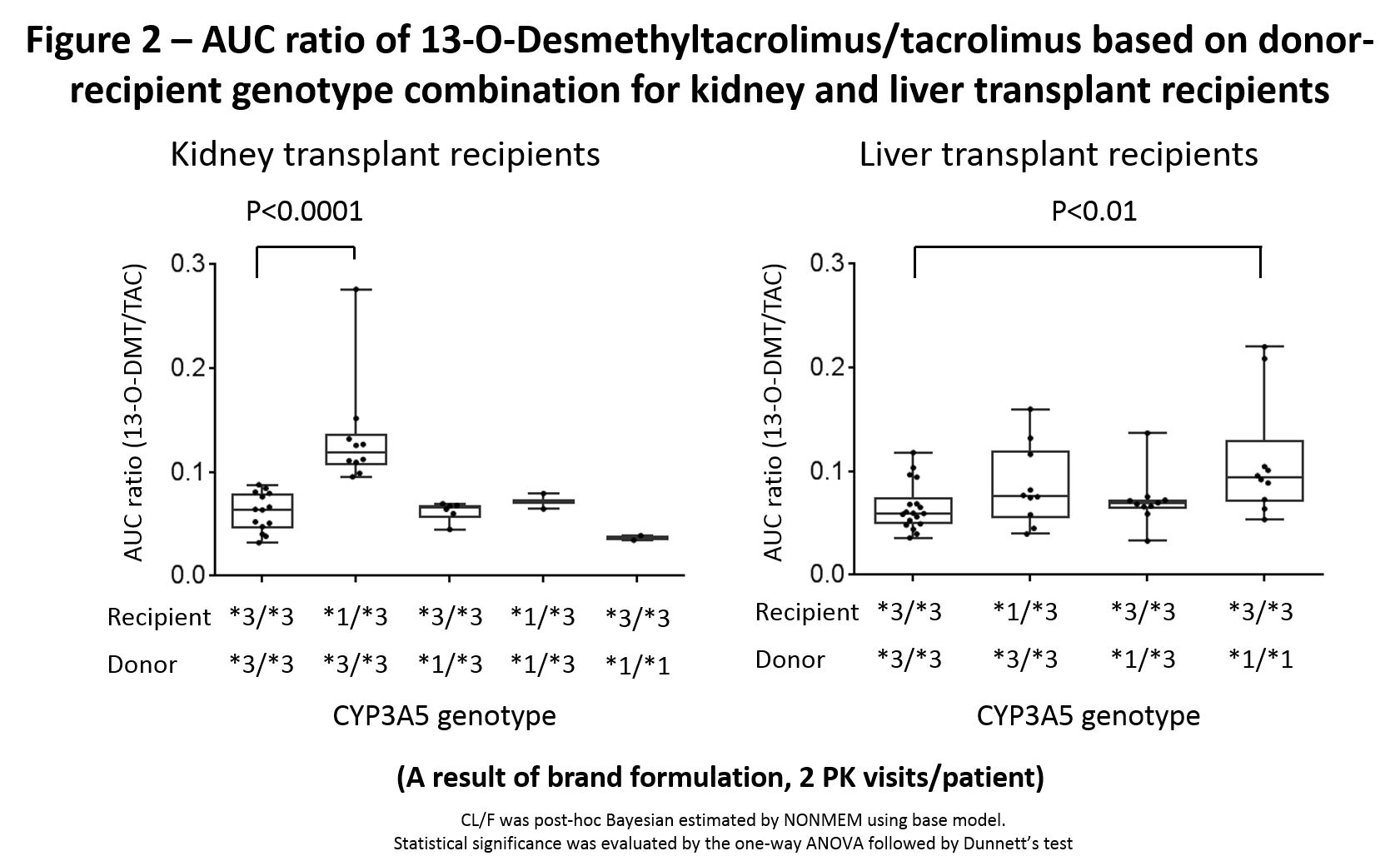 Conclusion: DG impacts tac and 13-ODMT PK in LT but not in KT. Kidney RG influenced CL/F, liver RG did not. CPIC guidelines recommend genotyping recipients for CYP3A5 to guide tac dosing and that in LT, DG may need to be considered. Our results support this statement. Prospective studies should evaluate the impact of recipient-DG on PK, clinical outcomes, biomarkers and relative importance of intestinal vs hepatic metabolism.
Conclusion: DG impacts tac and 13-ODMT PK in LT but not in KT. Kidney RG influenced CL/F, liver RG did not. CPIC guidelines recommend genotyping recipients for CYP3A5 to guide tac dosing and that in LT, DG may need to be considered. Our results support this statement. Prospective studies should evaluate the impact of recipient-DG on PK, clinical outcomes, biomarkers and relative importance of intestinal vs hepatic metabolism.
CITATION INFORMATION: Tremblay S, Fukuda T, Mizuno T, Woodle E, Abu Jawdeh B, Govil A, Christians U, Klawitter J, Vinks A, Alloway R. Impact of Donor Genotype on Recipient Tacrolimus Pharmacokinetics: The Liver Matters. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Tremblay S, Fukuda T, Mizuno T, Woodle E, Jawdeh BAbu, Govil A, Christians U, Klawitter J, Vinks A, Alloway R. Impact of Donor Genotype on Recipient Tacrolimus Pharmacokinetics: The Liver Matters. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-donor-genotype-on-recipient-tacrolimus-pharmacokinetics-the-liver-matters/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
