Impact of De Novo DSA Development and Management on Kidney Transplant Outcomes
1Pharmacy, Beth Israel Deaconess Medical Center, Boston, MA
2Transplant Institute, Beth Israel Deaconess Medical Center, Boston, MA
3Pathology, Beth Israel Deaconess Medical Center, Boston, MA.
Meeting: 2018 American Transplant Congress
Abstract number: D95
Keywords: Antibodies, Graft survival, High-risk, Rejection
Session Information
Session Name: Poster Session D: Kidney Complications: Late Graft Failure
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Multiple studies have shown that development of donor specific antibodies (DSAs) can have a negative impact on renal allograft outcomes resulting in decreased renal function, an increased incidence of antibody-mediated rejection (AMR) and an increase risk of allograft loss. Our center implemented a prospective DSA monitoring protocol in April 2014 to allow for earlier detection and management of de novo DSA (dnDSA) in at risk patients. The objective of the study was to evaluate the impact of dnDSA development and management on kidney transplant outcomes at 1 year post-transplant.
Methods: We conducted a single center, retrospective chart review of kidney transplant recipients from April 2014 through October 2016. The primary outcome was a composite endpoint of graft loss, patient death and rejection at 1 year. Secondary outcomes include contrubitng factors for dnDSA development, management of dnDSAs following identification, infection and malignancy.
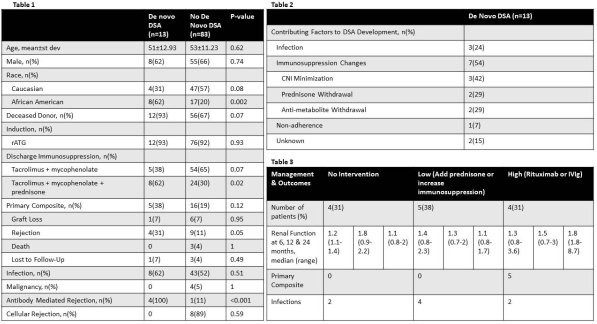
Results: Of the 96 kidney transplants included in the prospective monitoring protocol, 13 developed dnDSA. Key clinical characteristics and outcomes are depicted in Table 1. Patient characteristics were similar with regards to age, gender and donor type. Patients with dnDSAs were more likely to be African American and discharged on prednisone. There was no difference between groups in terms of the primary composite outcome (38% vs. 19%, p=0.21). Significantly more patients who developed dnDSA experienced rejection (31% vs. 11%, p=0.05) which was more often AMR (100% vs 11%, p<0.001). Immunosuppression changes was a major contributing factor (54%) to dnDSA development (Table 2).
Conclusion: Development of dnDSAs following kidney transplant are often related to a preceding reduction in immunosuppression and can lead to development of rejection. Adjustment of immunosuppression after identification of DSA may prevent rejection and graft loss. Standardized protocols for management of dnDSAs are warranted.
CITATION INFORMATION: Cote M., Pavlakis M., Cardarelli F., Pena J., Dinh A., Richards K., Joyal K., Rogers C. Impact of De Novo DSA Development and Management on Kidney Transplant Outcomes Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Cote M, Pavlakis M, Cardarelli F, Pena J, Dinh A, Richards K, Joyal K, Rogers C. Impact of De Novo DSA Development and Management on Kidney Transplant Outcomes [abstract]. https://atcmeetingabstracts.com/abstract/impact-of-de-novo-dsa-development-and-management-on-kidney-transplant-outcomes/. Accessed March 3, 2026.« Back to 2018 American Transplant Congress