Impact of Belatacept on Clinical Outcomes in Lung Transplant Patients with Antibody-Mediated Rejection
Duke University, Durham, NC
Meeting: 2022 American Transplant Congress
Abstract number: 1484
Keywords: Alloantibodies, Immunosuppression, Lung transplantation, Survival
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Antibody-mediated rejection (AMR) is cause of significant morbidity and mortality in lung transplant recipients (LTRs). An optimal therapeutic regimen is still lacking. Recent evidence suggests that belatacept, a costimulation blockade antibody, could prevent the generation of donor-specific antibodies (DSAs) in kidney transplant recipients. However, its use in LTRs has not been evaluated. We hypothesized that a multimodal approach including belatacept could have a beneficial impact on LTRs with AMR.
*Methods: In the current study, we retrospectively analyzed the clinical outcomes of LTRs with AMR who received as first-line intervention a combination of plasmapheresis (PLEX), IVIG, rituximab, and carfilzomib with belatacept (group 1, n=10) or without (group 2, n=9). LTRs who received previous therapeutic interventions were excluded. Changes in forced expiratory volume at 1 second (FEV1) and circulating DSAs 90 days post-treatment were collected. Kaplan-Meier method was used to analyze survival using composite endpoint of death or re-transplantation at 30, 90 days, and 1 year.
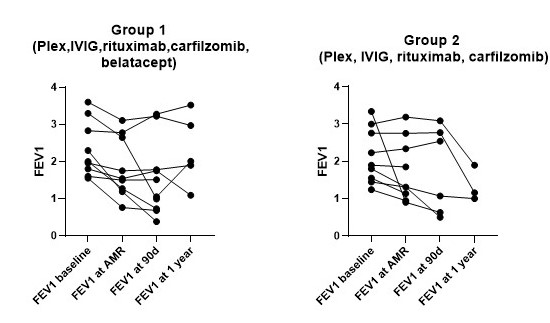
*Results: The two groups were similar in age, sex, and underlying disease as shown in table 1. All patients received a bilateral lung transplant (BOLT) and were on triple-drug maintenance immunosuppression. No difference in pre-transplant calculated panel-reactive antibody was noted. There was no difference in FEV1 at baseline (p=0.6) and at AMR diagnosis (figure 1) (p= 0.8, respectively). AMR was diagnosed at a median of 14.5 months (interquartile range[IQR], 2-26) in group 1 and of 16 months (IQR, 7-53) in group 2. All LTRs developed class II DSAs. There was no significant difference in FEV1 at 90 days post-treatment between the 2 groups (figure 1) (p=0.6). In each group 3 patients obtained clearance of circulating DSAs at 90 days. There was no difference in survival between the 2 groups (log-rank p= 0.3). A total of 7 LTRs developed chronic lung allograft dysfunction (CLAD), 4 died and 3 received second BOLT (table 1)
*Conclusions: Our study suggests that belatacept might not have a significant impact on the management of AMR in LTRs. However, additional prospective studies are needed to define optimal therapeutic interventions.
| Plex, IVIG, Rituximab, Carfilzomib + Belatacept n=10 | Plex, IVIG, Rituximab, Carfilzomib n=9 | |
| Gender | M=8 F=2 | M=3 F=6 |
| Age at Transplant (mean,range) | 52.4 (19-77) | 52.6 (17-69) |
| Primary Disease •Restrictive, •Obstructive | 6, 4 | 4, 5 |
| AMR diagnosis months from txp (median,IQR) | 14.5 (2-26) | 16 (7-53) |
| Mean FEV1 (L), •Baseline, •AMR diagnosis, •90 days | 2.33, 1.83, 1.53 | 2.14, 1.65, 1.76 |
| Patients (n) with Clearance DSA at 90 days | 3 | 3 |
| Death or Retransplantation •30 days, •90 days, •1 year | 0, 1, 3 (2 retransplants) | 0, 2, 3 (1 retransplant) |
To cite this abstract in AMA style:
Zaffiri L, Hallak R, Leonard S, Neely M, Hulber A, Berry H, Young KA, Reynolds JM. Impact of Belatacept on Clinical Outcomes in Lung Transplant Patients with Antibody-Mediated Rejection [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-belatacept-on-clinical-outcomes-in-lung-transplant-patients-with-antibody-mediated-rejection/. Accessed March 6, 2026.« Back to 2022 American Transplant Congress