Impact of Belatacept Conversion on Renal Function and Donor Specific Antibodies in Renal Transplant Recipients
Northwestern Memorial Hospital, Chicago, IL
Meeting: 2022 American Transplant Congress
Abstract number: 851
Keywords: Calcineurin, Glomerular filtration rate (GFR), Immunosuppression, Renal dysfunction
Topic: Clinical Science » Kidney » 47 - Kidney Complications: Immune Mediated Late Graft Failure
Session Information
Session Name: Kidney Complications: Immune Mediated Late Graft Failure
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Although associated with nephrotoxicity, calcineurin inhibitors (CNI) have historically been the cornerstone of immunosuppression. Belatacept, a co-stimulation blocker, is FDA-approved for de novo use post-renal transplant. Transplant centers have since adopted conversion strategies with the goal of preserving renal function while minimizing or discontinuing CNIs. Belatacept has recently demonstrated improvement or stabilization of pre-existing donor specific antibodies (DSA).
*Methods: This was a retrospective study of adult renal transplant recipients to assess the impact of conversion to belatacept on renal function and DSA. Patients that received a multi-organ transplant or did not reach maintenance dosing for belatacept were excluded. Evaluated outcomes included estimated glomerular filtration rate (eGFR), biopsy proven acute rejection (BPAR), and DSA titers pre and post-conversion.
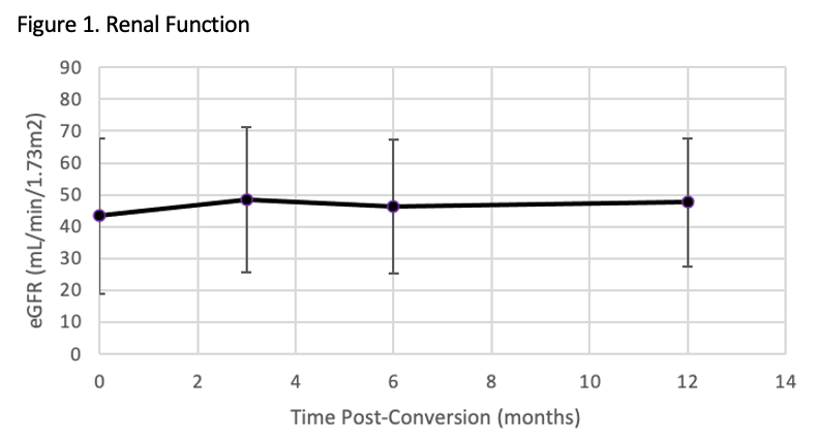
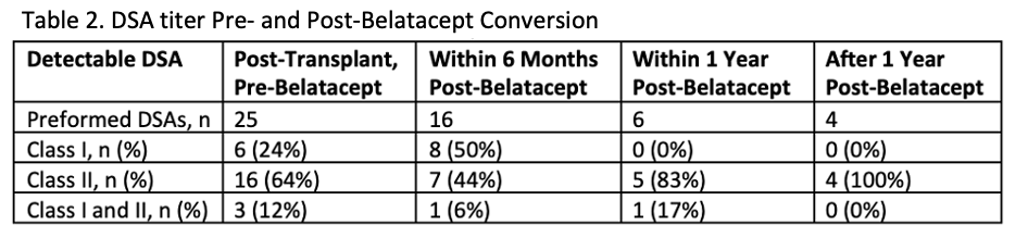
*Results: One hundred and twenty-six patients were included. Prior to belatacept conversion, 80% of patients were on a CNI based regimen and 15% were on either a mammalian target of rapamycin inhibitor (mTORi) based regimen or combination of CNI and mTORi regimen. The other 5% of patients were on an antimetabolite +/- prednisone. Reasons for conversion included CNI toxicity (45%), antimetabolite or mTORi toxicity or avoidance (35%), or need for escalation of immunosuppression (20%). An average improvement in eGFR of 5.09 mL/min/1.73m2, 5.51 mL/min/1.73m2, and 6.85 mL/min/1.73m2 at 3, 6 and 12 months post-conversion, respectively. Thirteen patients with detectable DSA prior to conversion experienced either undetectable, decreased or stable titers after conversion. Two patients developed de novo DSA post belatacept conversion. Thirty-three patients experienced borderline or more severe rejection episodes post belatacept conversion.
*Conclusions: Conversion to belatacept for maintenance immunosuppression in renal transplant patients resulted in stable renal function as measured by eGFR. The efficacy of belatacept on long-term preservation of renal function and DSA will need to be evaluated in larger clinical trials that follow patients over an extended period of time.
To cite this abstract in AMA style:
Brown ER, Cunningham K, Paplaczyk K, Dagostino C, Kane C, Shetty A. Impact of Belatacept Conversion on Renal Function and Donor Specific Antibodies in Renal Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-belatacept-conversion-on-renal-function-and-donor-specific-antibodies-in-renal-transplant-recipients/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress