Impact of a Pharmacist Led Outpatient Tacrolimus Monitoring Protocol in Renal Transplant Recipients
Ohio State University Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: D-098
Keywords: Calcineurin, Immunosuppression, Kidney, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
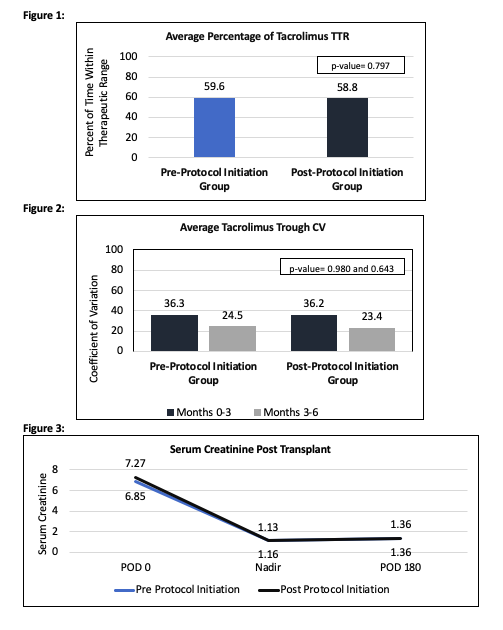
*Purpose: The purpose of this study is to compare the time within therapeutic range (TTR) of tacrolimus (TAC) before and after the initiation of a pharmacist led TAC management protocol. Outpatient TAC levels outside of goal range are sent to the transplant (TXP) pharmacists for primary review and dose adjustments. Secondary outcomes include coefficient of variation (CV) of TAC levels, number of TAC dose changes, serum creatinine at postoperative day 180, development of donor specific antibodies (DSAs) and incidence of biopsy proven acute rejection (BPAR).
*Methods: This was a single-center, retrospective review of adult kidney transplant recipients (KTR) who underwent TXP between September 1, 2017 to March 31, 2019 and received de novo immediate release TAC. KTR who underwent TXP prior to the pharmacist led protocol initiation in September 2018 were compared to KTR who were transplanted post initiation of the protocol. Patients were followed for 6 months. Exclusion criteria included: deviation from standard TAC protocol, formulation changes, multi-organ transplant, strong CYP3A4 inducers or inhibitors, and those who had detectable cytomegalovirus, Epstein Barr virus, or BK. TTR of TAC was calculated using the Rosendaal method.
*Results: A total of 78 KTR were included (39 patients in each arm) with a mean follow-up of 159.74 days (108.3-174.3 days). The average TTR in the first 6 months post KT was 59.6% in the pre-protocol group and 58.8% in the post-protocol group (Figure 1, p=0.797). The average CV during the first 3 months post-TXP and months 3 through 6, was 36.3 and 24.5 in the pre-protocol group and 36.2 and 23.4 in the post-protocol group respectively (Figure 2, p=0.980 and 0.643). The pre-protocol group was admitted for a total of 121 days (average 3.102 days per patient) versus 58 days (average 1.49 days per patient) in the post-protocol group. There was no difference in the average number of TAC dose changes made during follow up (6 vs 5.46 in pre-protocol and post-protocol group respectively). In the pre-protocol group, 3 patients developed DSAs within 180 days of TXP versus 1 in the post-protocol group. No patient in either group developed BPAR. Serum creatinine did not differ between the groups at postoperative day 180 with an average serum creatinine 1.36 (Figure 3).
*Conclusions: There was no significant difference in TTR or CV of TAC levels after the pharmacist led protocol was initiated. Pharmacists were equally as efficacious at maintaining TAC in TTR after the initiation of the protocol when compared to prior management.
To cite this abstract in AMA style:
Owen KL, Palettas M, Winters H, Pesavento TE, Rajab A, Witkowsky O. Impact of a Pharmacist Led Outpatient Tacrolimus Monitoring Protocol in Renal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-a-pharmacist-led-outpatient-tacrolimus-monitoring-protocol-in-renal-transplant-recipients/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress