Impact of a De Novo Belatacept Based Immunosuppressive Regimen on Kidney Transplant Outcomes
Massachusetts General Hospital Transplant Center, Boston, MA
Meeting: 2020 American Transplant Congress
Abstract number: C-113
Keywords: Immunosuppression, Kidney, Rejection
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Use of anti-thymocyte globulin (rabbit) (rATG) induction with belatacept/mycophenolate (MPA)/prednisone based immunosuppression (IS) has led to a reduction in rejection compared to that seen with basiliximab. This study sought to determine if use of belatacept with maintenance everolimus and prednisone would improve efficacy and maintain safety.
*Methods: Retrospective, single-center analysis of 33 EBV seropositive kidney transplant recipients (KTRs) with at least 6 months of follow up (f/u). KTRs received 4.5 mg/kg rATG or basiliximab induction, FDA approved dosing of belatacept, and MPA or everolimus with prednisone.
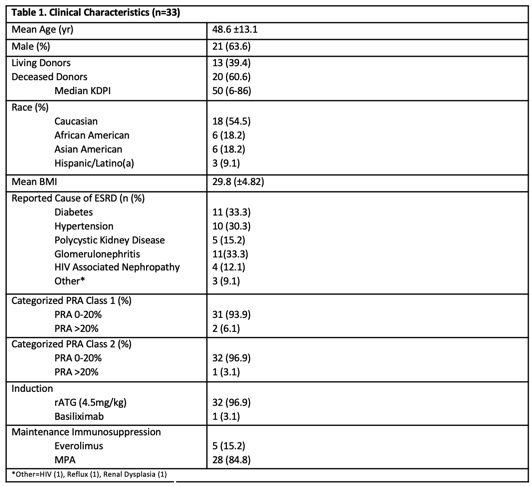
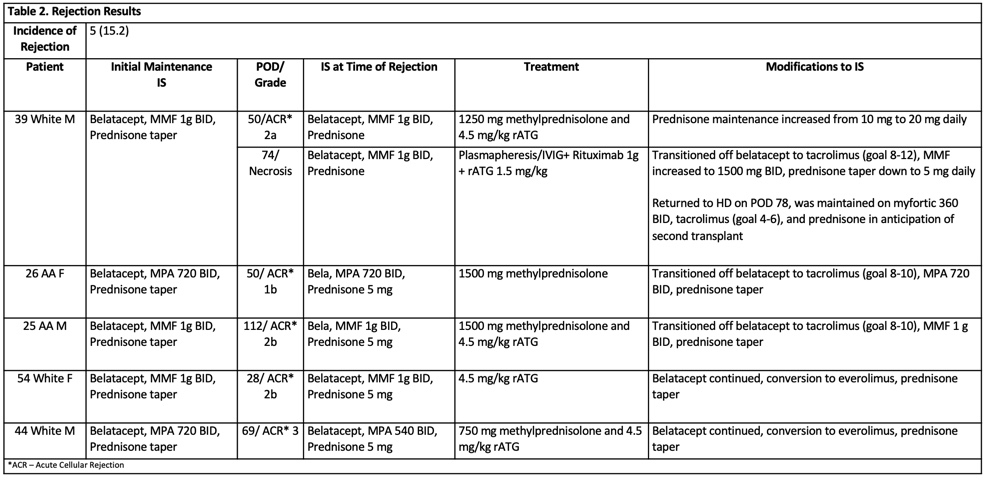
*Results: Clinical characteristics and details of rejection treatment and management are presented in Table 1 and Table 2. All patients were alive at month 6 and at the end of study follow up (67% with 12 month f/u). One patient experienced graft loss on POD 78. Median eGFR in those with functioning grafts w/o rejection was 62 and with rejection was 61.4. Ten patients developed a viral infection (CMV, BKV, Influenza, HSV) and 8 developed a bacterial infection. One patient developed squamous cell carcinoma.
*Conclusions: A de novo belatacept based regimen with rATG induction and maintenance MPA with prednisone resulted in an ACR rate consistent with previous studies which is lower than regimens including basiliximab induction, though still higher than typically seen with tacrolimus-based regimens. As there were only five patients treated with de novo belatacept in combination with everolimus and prednisone, further studies are needed to evaluate the impact of this regimen on safety and outcomes.
To cite this abstract in AMA style:
Joyal K, Vanhove T, Schreiber B, Cote M, Shao S, Elias N, Gilligan H, Safa K, Heher E, Williams W, Rogers C. Impact of a De Novo Belatacept Based Immunosuppressive Regimen on Kidney Transplant Outcomes [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-a-de-novo-belatacept-based-immunosuppressive-regimen-on-kidney-transplant-outcomes/. Accessed July 14, 2025.« Back to 2020 American Transplant Congress