Immunotherapy of Dc-cik Cells in Patient with Hepatocellular Carcinoma Recurrence Following Liver Transplantation
China Medical University Hospital, Taichung, Taiwan
Meeting: 2021 American Transplant Congress
Abstract number: 1114
Keywords: Hepatocellular carcinoma, Liver transplantation, Safety
Topic: Clinical Science » Liver » Liver: Hepatocellular Carcinoma and Other Malignancies
Session Information
Session Name: Liver: Hepatocellular Carcinoma and Other Malignancies
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Dendritic cells (DCs) and cytokine-induced killer (CIK) cells, components of anti-cancer therapy, have shown clinical benefits and potential to overcome chemotherapeutic resistance in some cancer patients. However, only fewer experience published about the safety and efficacy of DC/CIK in the transplant patients. We would like to share our experiences of DC/CIK therapy in the transplant patients.
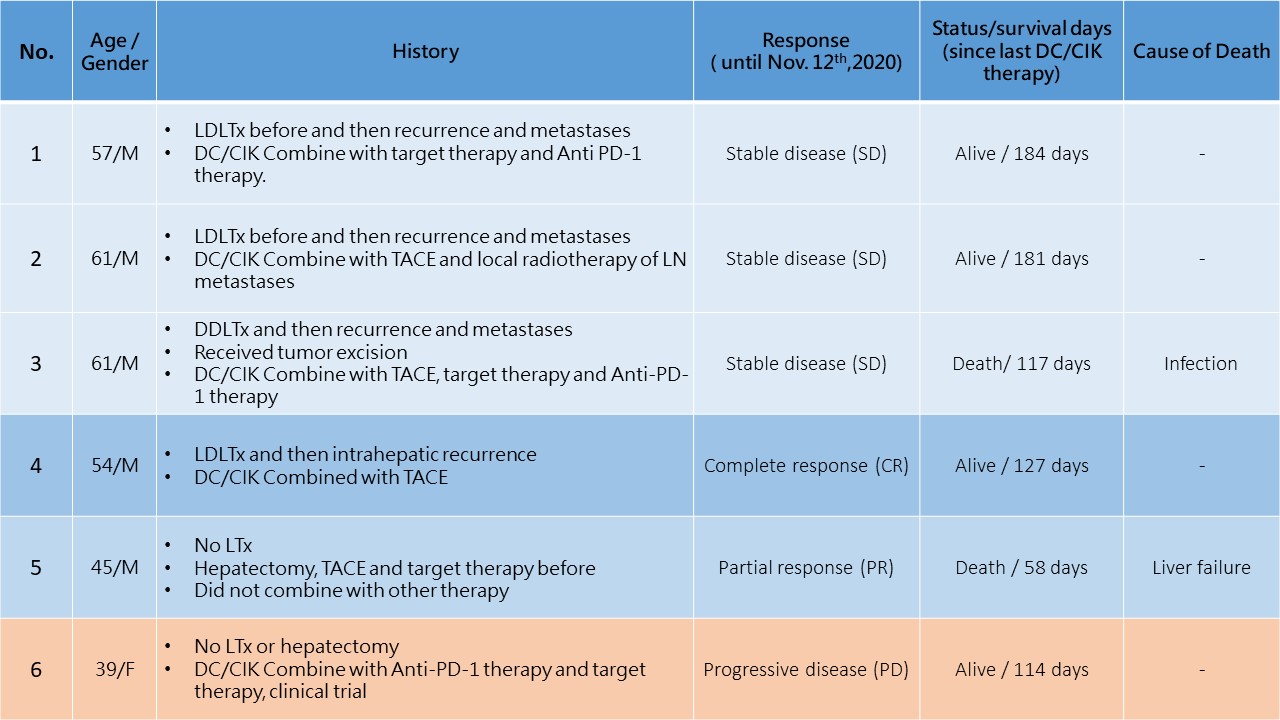
*Methods: Until November 12th, 2020, 8 patients receiving DC/CIK therapy for hepatocellular carcinoma recurrence or metastasis (Table 1). 6 patients already received liver transplantation, but another 2 patients did not. 6 patients already completed DC/CIK treatment courses and another 2 patients are processing. Almost all patients even received other therapies, like checkpoint inhibitor, target therapy, trans-arterial chemoembolization or radiotherapy. Images and laboratory data are used to evaluate the patient’s response.
*Results: Of 6 patients with complete DC/CIK therapy, the survival days are 58 days to 184 days. Images and laboratory data approve 1 patient with complete responses (CR). 1 patient is approved as partial response (PR), and 3 patients stay with stable disease (SD). 1 patient is approved as progressive disease (PD). 2 patients already died because of infection and disease progression later. No patient develops any rejection or cytokine release syndrome.
*Conclusions: Multidisciplinary therapy is a favored treatment option for HCC patients now right, like checkpoint inhibitor therapy and target therapy together or local treatment and target therapy. However, checkpoint inhibitor is still relatively contra-indication to the transplant patient because of potential graft rejection. Thus, DC/CIK could be another alternative therapy. In our experiences, DC/CIK is safe and has potential efficacy in the transplant patient.
To cite this abstract in AMA style:
Hsu S, Jeng L, Yang H, Thorat A. Immunotherapy of Dc-cik Cells in Patient with Hepatocellular Carcinoma Recurrence Following Liver Transplantation [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/immunotherapy-of-dc-cik-cells-in-patient-with-hepatocellular-carcinoma-recurrence-following-liver-transplantation/. Accessed March 5, 2026.« Back to 2021 American Transplant Congress