Immunogenicity of a 3rd Dose of Mrna Covid-19 Vaccine in Kidney Transplant Recipients Who Failed to Respond to 2 Prior Doses
W. Werbel1, A. Karaba1, T. Chiang1, T. Liang1, A. Massie1, M. Bettinotti1, W. Clarke1, N. Watson2, A. Tobian1, C. Durand1, N. Bridges2, D. Rostrosen2, C. Larsen3, P. Heeger4, D. Segev1
1Johns Hopkins, Baltimore, MD, 2NIAID, Bethesda, MD, 3Emory University, Atlanta, GA, 4Mt. Sinai School of Medicine, New York, NY
Meeting: 2022 American Transplant Congress
Abstract number: 687
Keywords: Antibodies, COVID-19, Kidney transplantation, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) I
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Kidney transplant recipients (KTRs) have diminished immune response and protection after 2-dose mRNA COVID-19 vaccination. It is unknown if additional doses improve neutralization of variants of concern (VOC) in KTRs with prior poor seroresponse.
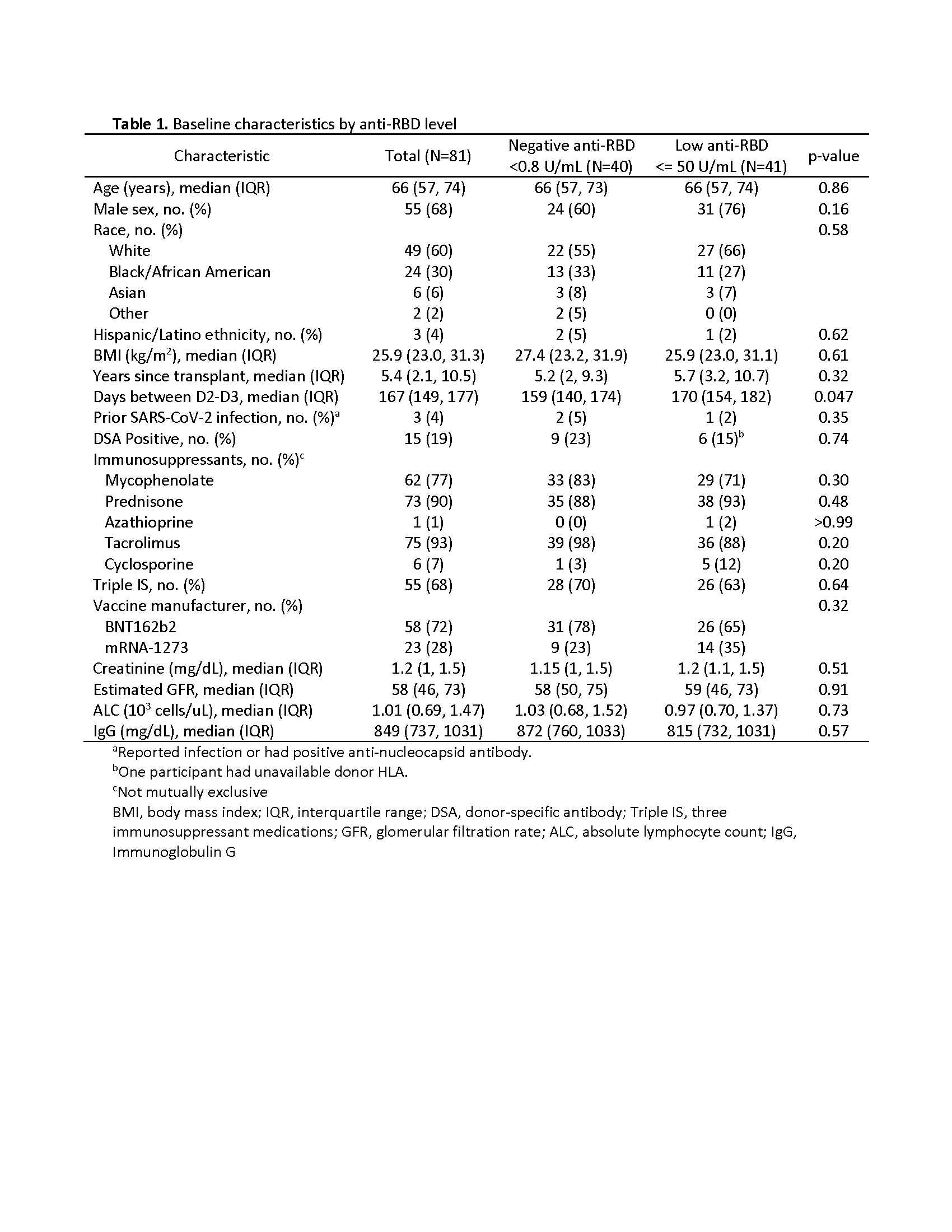
*Methods: Adult KTRs with negative (<0.8 U/mL) or low (≤50 U/ml) anti-RBD Ig (Roche Elecsys anti-SARS-CoV-2-S) after 2-dose mRNA series were given a homologous 3rd dose (D3). Anti-RBD and VOC surrogate neutralization (%ACE2i) were measured 30 days post D3; responses were stratified by baseline anti-RBD. Reactogenicity, serial SARS-CoV-2 swabs, and donor-specific antibody (DSA) were assessed.
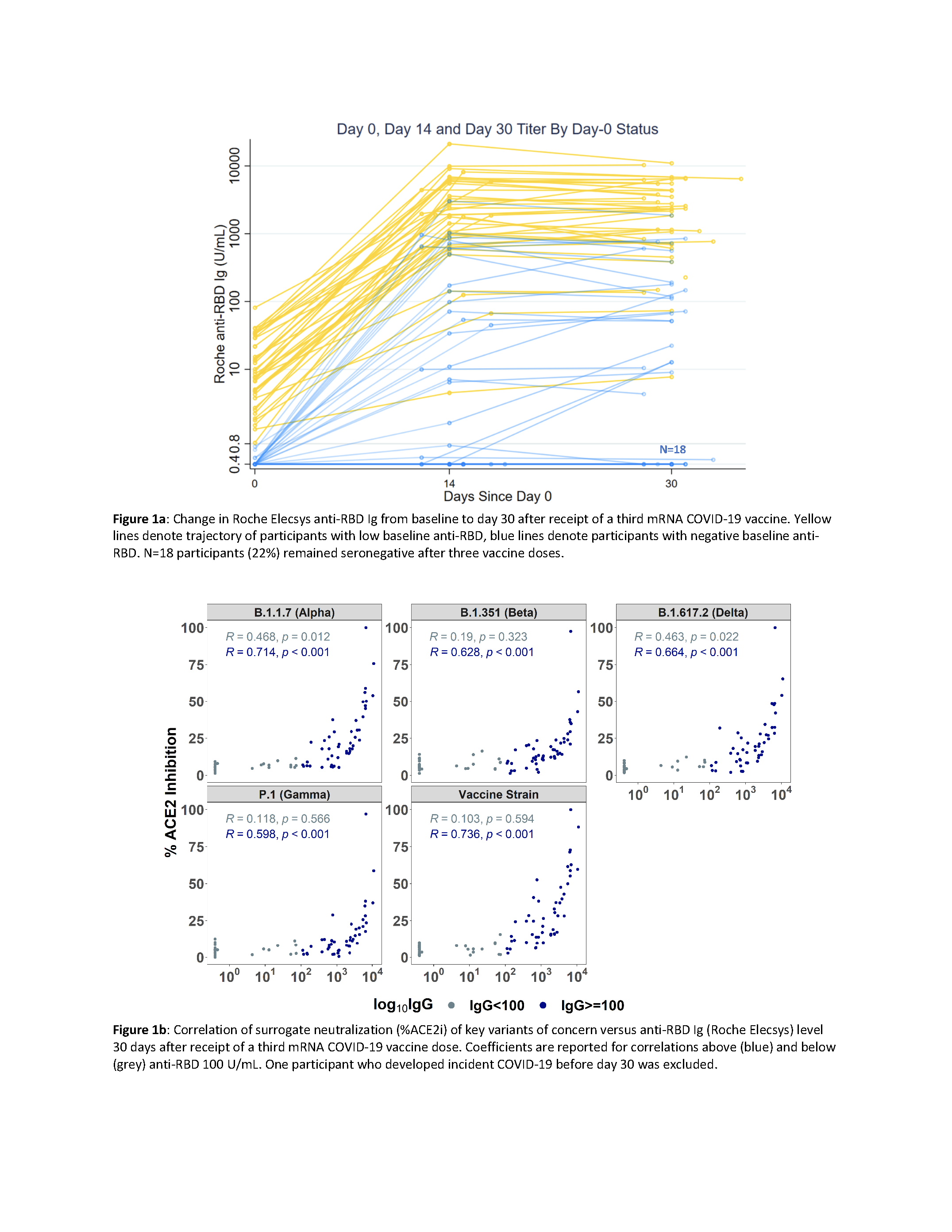
*Results: 81 KTRs (50% negative anti-RBD) received D3 (72% BNT162b2, 28% mRNA-1273) at median 167 days post D2 (Table). Median (IQR) anti-RBD increase was 410 (8-2309) U/mL with 69% (40% negative vs 98% low anti-RBD) achieving day 30 anti-RBD >50 U/ml (Fig1a). 22% remained seronegative. Non-response was associated with lower baseline lymphocyte count (median 770 vs 1160 cells/uL; p=0.05) and IgG (median 779 vs 979 mg/dL; p<0.01), but not demographics, vaccine, or immunosuppressives. Median (IQR) delta variant %ACE2i increased from 6% (3-7) to 10% (4-22) (p<0.001), a 1% (0-5) increase in negative vs 13% (5-25) in low anti-RBD. %ACE2i was linearly associated with anti-RBD ≥100 U/mL (all VOC shown in Fig1b); 64% of KTRs with anti-RBD ≥250 U/mL had delta %ACE2i >20. There were 3 cases of mild-moderate COVID-19 ≥7 days post-D3, with pre-infection anti-RBD <0.4, 22, 76 U/mL and delta %ACE2i 6, 9, and 16, respectively. There was no acute rejection, nor increased or de novo DSA.
*Conclusions: A 3rd mRNA vaccine dose increased anti-RBD and VOC neutralization in KTRs without inducing clinical alloimmunity, yet 45% with negative baseline anti-RBD remained seronegative without delta variant neutralization. Trials are ongoing to test immune response augmentation in this subgroup via temporary immunosuppression reduction or heterologous boosting.
To cite this abstract in AMA style:
Werbel W, Karaba A, Chiang T, Liang T, Massie A, Bettinotti M, Clarke W, Watson N, Tobian A, Durand C, Bridges N, Rostrosen D, Larsen C, Heeger P, Segev D. Immunogenicity of a 3rd Dose of Mrna Covid-19 Vaccine in Kidney Transplant Recipients Who Failed to Respond to 2 Prior Doses [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/immunogenicity-of-a-3rd-dose-of-mrna-covid-19-vaccine-in-kidney-transplant-recipients-who-failed-to-respond-to-2-prior-doses/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress