Immunobiogram, a New IVD Immunoassay to Test the Sensitivity Profile of Kidney Transplant Recipients to Immunosuppressive Drugs: Further Results from BH-Pilot Study
1Nephrology Department, La Paz Hospital, Madrid, Spain, 2Nephrology Department, Puerta de Hierro Mahadahonda Hospital, Madrid, Spain, 3Adamas Engineering Consulting, Madrid, Spain, 4I+D, Biohope, Madrid, Spain, 5Medical, Biohope, Madrid, Spain, 6CEO, Biohope, Madrid, Spain
Meeting: 2019 American Transplant Congress
Abstract number: B45
Keywords: Immunosuppression, Kidney transplantation, Prediction models, T cell activation
Session Information
Session Name: Poster Session B: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: IMBG is an immunoassay developed by Biohope to measure in vitro PBMC sensitivity/resistance profile to a panel of immunosuppressive drugs (IS) in Kidney Transplant recipients (KT). Advanced neural network and clustering techniques were developed to automatically classify patients in IS sensitivity grades.
*Methods: IMBG comprises a biotechnology process and a data interpretation tool.
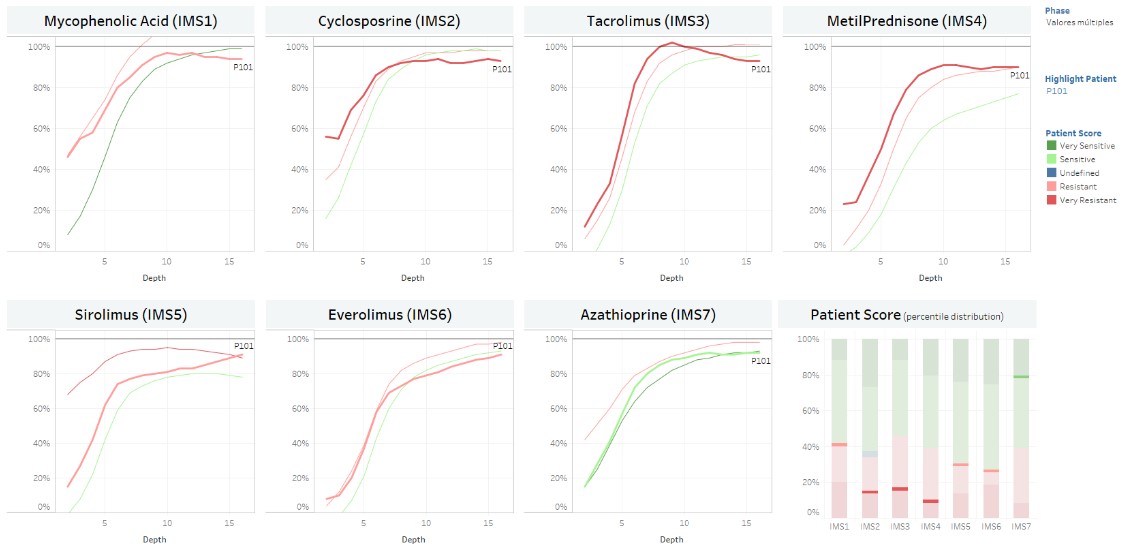
IMBG is based on PBMCs 3D culture in semi-solid matrix submitted to immune stimulation and has been presented elsewhere (M. Di Scala et al BST 2018). The system reveals the capacity of an IS gradient to inhibit the activation cells state, which can be translated into a dose/response sigmoid curve. BH-pilot study is an observational PoC clinical study which included 70 patients at least 1 year after KT, who were classified into immunological high-risk, standard and low-risk patients. A mathematical score per IS and an expert-based independent evaluation of all IS responses per patient were performed. A strong correlation between IS resistance at IMBG and poorer clinical outcome was shown. Here we present the advanced neural network and clustering techniques developed to automatically classify patients IMBG in sensitivity grades (per IS and globally).
*Results: The neural network patient classification was trained with raw data from fluorimeter of 50 patients with equal results in mathematical scoring and expert evaluation and then applied for reclassification of whole sample. 55% of clinically high-risk patients showed an IMBG low-sensitivity profile and 78% of low-risk patients a high-sensitivity profile to the IS they were taking.
Cluster analysis used normalized IMBG curves and key curve parameters were described. Automatized clustering methods were applied resulting in Very Resistant/Resistant/Sensitive/Very Sensitive groups, providing an individual score per patient and IS.
*Conclusions: IMBG provides an automatized method to quantitatively measure patients´ PBMC sensitivity profile to a panel of immunosuppressive drugs in kidney transplant recipients
To cite this abstract in AMA style:
Jimenez C, Portoles JM, Blánquez D, Arribas L, Ortega A, Diez T, Portero I. Immunobiogram, a New IVD Immunoassay to Test the Sensitivity Profile of Kidney Transplant Recipients to Immunosuppressive Drugs: Further Results from BH-Pilot Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/immunobiogram-a-new-ivd-immunoassay-to-test-the-sensitivity-profile-of-kidney-transplant-recipients-to-immunosuppressive-drugs-further-results-from-bh-pilot-study/. Accessed February 19, 2026.« Back to 2019 American Transplant Congress