Immune Checkpoint Inhibitor Use in Kidney Transplant Recipients: Rejection Risk, Prevention and Transcriptional Signature in Allograft
1Brigham and Women's Hospital, Boston, MA, 2University of Alberta, Edmonton, AB, Canada
Meeting: 2020 American Transplant Congress
Abstract number: B-210
Keywords: Co-stimulation, Kidney transplantation, Malignancy, Rejection
Session Information
Session Name: Poster Session B: PTLD/Malignancies: All Topics
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Immune checkpoint inhibitor (ICI) use has changed the landscape of cancer treatment. Several cases of acute rejection in use of ICI have been reported, while others reported successful management. The prevalence, risk factors and mechanism of acute rejection, and how to best tailor immune suppression have not been fully understood.
*Methods:
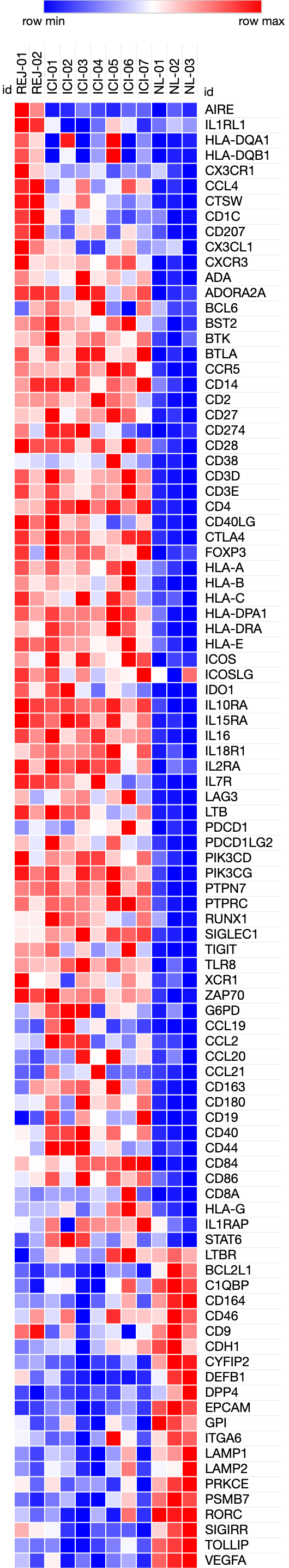
*Results: We conducted a systematic literature review and calculated the rejection risk in different ICI regimen. Of 48 total cases of kidney transplant recipients who received ICI, 18 cases (37.5%) experienced rejection. The rejection rate was highest in Nivolumab (anti-PD-1, 50.0%) and combination ICI (50.0%), followed by pembrolizumab (anti-PD-1, 29.4%) and ipilimumab (anti-CTLA4, 22.2%). Concomitant use of mTOR inhibitor (everolimus or sirolimus) did not have significant effect rejection rate (odds ratio 0.65 [95% CI: 0.18-2.20]). In our institution, we successfully treated 3 cases of skin squamous cell carcinoma in kidney transplant recipients with pembrolizumab or cemiplimab (anti-PD-1) without rejection event by using concomitant low-dose steroid pulses upon ICI cycle (modified protocol from NEJM 2017). In contrast, we had experienced one case of mixed T-cell mediated and antibody-mediated rejection when pulse steroid was not used. To investigate the molecular mechanism of ICI-associated immune rejection, we performed targeted gene expression analysis of kidney biopsy samples (ICI-rejection (n=2), ICI-associated acute interstitial nephritis in native kidneys (n=7), normal controls (n=3) using Nanostring platform, which allows us to quantify the expression level of ~770 immune-pathway related genes. Gene expression analysis showed that ICI-associated rejection shared similar gene expression signature with ICI-associated acute interstitial nephritis. In both groups, T-cell activation markers, HLA molecules and co-inhibitory receptors are upregulated. (Figure)
*Conclusions: ICI use in kidney transplant recipient is associated with high risk of rejection, but concomitant use of small-dose of steroid could be a potential strategy to mitigate acute rejection. Transcriptional analysis of ICI-associated rejection highlighted T-cell activation and cytokine pathway.
To cite this abstract in AMA style:
Murakami N, Reid G, Weins A, Adam B, Mengel M, Riella L. Immune Checkpoint Inhibitor Use in Kidney Transplant Recipients: Rejection Risk, Prevention and Transcriptional Signature in Allograft [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/immune-checkpoint-inhibitor-use-in-kidney-transplant-recipients-rejection-risk-prevention-and-transcriptional-signature-in-allograft/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress