How Safe is Crossing the ABO Blood Group Barrier? A Meta-Analysis to Determine the Additive Risk of ABO-Incompatible Kidney Transplantation
Nephrology and Kidney Transplantation, Erasmus Medical Center, Rotterdam, Netherlands.
Meeting: 2018 American Transplant Congress
Abstract number: 326
Keywords: Graft survival, Kidney transplantation, Mortality, Plasmapheresis
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Desensitization
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Room 4B
Background ABO blood group-incompatible (ABOi) kidney transplantation is considered a safe procedure, with non-inferior outcomes in large cohort studies. Its contribution to living kidney transplantation programs is substantial and growing. The objective of this meta-analysis was to systematically investigate outcomes in ABO-incompatible kidney transplant recipients compared to center-matched ABO blood group-compatible (ABOc) control patients.
Methods Comprehensive searches were conducted in Embase, Medline, Cochrane, Web-of-Science and Google Scholar. MOOSE study guidelines for observational studies and Newcastle Ottawa bias scale were implemented to assess studies. Meta-analysis was performed using Review Manager 5.3. A subgroup analysis on antibody removal technique was performed.
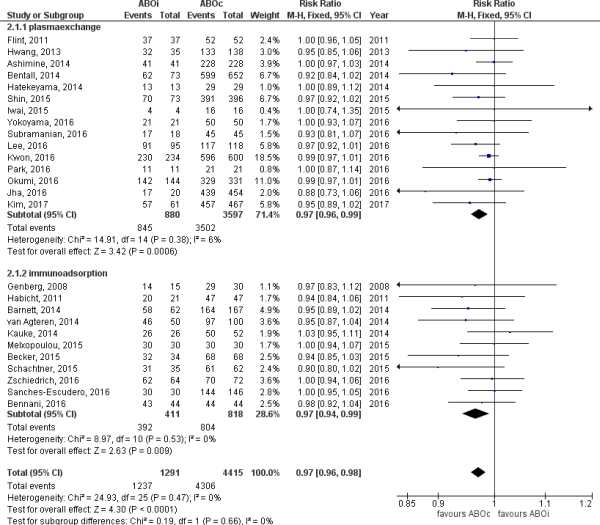
Results After identifying 2728 studies addressing ABOi kidney transplantation, 26 studies were included, describing 1346 unique ABOi patients and 4943 ABOc controls. Baseline patient characteristics revealed no significant differences in immunological risk parameters. Clinical heterogeneity of studies was very low (I2 0% for graft and patient survival). ABOi patients had very good one-year graft survival (95[middot]82%), albeit worse than ABOc controls (RR 0[middot]97, CI 0[middot]96-0[middot]98, p<0.0001). 49% of reported causes of death in ABOi were of infectious origin, versus only 13% in ABOc patients (p=0.02). ABMR (3[middot]86, CI 2[middot]05-7[middot]29, p<0[middot]00001), severe non-viral infection (1[middot]41, CI 1[middot]11-1[middot]78, p=0.[middot]005) and bleeding ABOc (1[middot]92, CI 1[middot]36-2[middot]72, p=0[middot]0002) were more common after ABOi transplantation. Plasmapheresis versus immunoadsorption did not affect outcomes.
Conclusion ABOi kidney transplant recipients have very good outcomes albeit inferior to center-matched ABOc control patients. The low clinical heterogeneity of included studies and similar baseline characteristics strengthen our findings and should encourage the use of kidney exchange programs.
Funding None.
Figure 1.
CITATION INFORMATION: de Weerd A., Betjes M. How Safe is Crossing the ABO Blood Group Barrier? A Meta-Analysis to Determine the Additive Risk of ABO-Incompatible Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Weerd Ade, Betjes M. How Safe is Crossing the ABO Blood Group Barrier? A Meta-Analysis to Determine the Additive Risk of ABO-Incompatible Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/how-safe-is-crossing-the-abo-blood-group-barrier-a-meta-analysis-to-determine-the-additive-risk-of-abo-incompatible-kidney-transplantation/. Accessed March 2, 2026.« Back to 2018 American Transplant Congress