HLA-DR/DQ Molecular Mismatch Dictates Early Risk of De Novo Donor-Specific Antibody Development and Offers a Personalized Approach to Immunosuppression Management in Kidney Transplantation
1University of Colorado, Aurora, CO, 2University of Manitoba, Winnipeg, MB, Canada, 3Clinimmune, Aurora, CO
Meeting: 2020 American Transplant Congress
Abstract number: 204
Keywords: Epitopes, HLA antibodies, HLA matching, Rejection
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes II
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: Clinicians have few tools to predict the risk of alloimmune injury in a patient undergoing renal transplantation, as traditional demographic or clinical variables lack precision. HLA-DR/DQ molecular mismatch may be an important indicator of immunologic risk that could guide immunosuppressive therapy but has only been tested in a single Canadian cohort. The purpose of this study was to evaluate the utility of molecular mismatch to predict the development of de novo donor specific antibodies (dnDSA) during the first year of transplant and to explore how differences in tacrolimus (TAC) exposure may modulate this risk.
*Methods: We performed high-resolution Class II HLA typing to determine HLA-DR and -DQ eplet mismatches between 444 renal transplant donor and recipient pairs at University of Colorado between 2007 and 2013. Previously defined single molecule eplet mismatch thresholds were used to stratify recipients into low (N=119), intermediate (N=153), and high-risk (N=172) categories. All patients were initiated and maintained on tacrolimus and were screened for dnDSA at months 1, 6, 12 and when clinically indicated. Tacrolimus troughs from 1 week to 12 months were used and troughs after dnDSA were excluded.
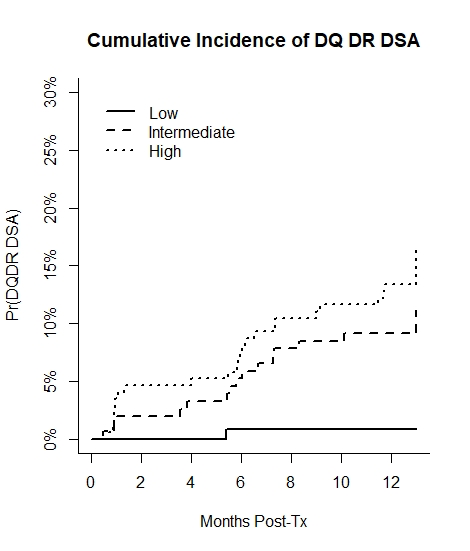
*Results: In this independent cohort the preset cutoffs defining the risk thresholds were first validated with AUCs of 0.87 and 0.82 for HLA-DR and HLA-DQ dnDSA, respectively. Only one low risk patient developed dnDSA (Figure) despite many having traditional measures of alloimmune risk. Compared to low risk patients, there was a graded increase in risk of DR/DQ dnDSA by 1 year in intermediate (HR 15.39, 95% CI 2.01-118.09, p=0.009) and high-risk (HR 23.81, 95% CI 3.17-178.66, p=0.002) categories, adjusting for age, deceased-donor, and induction. Intermediate and high risk patients with a mean tacrolimus < 6 ng/ml versus > 6 ng/ml had increased risk of DR/DQ dnDSA at 1 year (HR 2.35, 95% CI 1.05-5.28, p=0.04), while no low risk patients with a mean TAC < 6 ng/ml (13%) experienced DR/DQ dnDSA.
*Conclusions: HLA molecular mismatch represents a reproducible, objective, and clinically relevant tool to stratify patients by alloimmune risk and help guide personalized immunosuppression management.
To cite this abstract in AMA style:
Davis S, Wiebe C, Campbell K, Anobile C, Aubrey M, Wiseman A, Pomfret E, Nickerson P, Cooper J. HLA-DR/DQ Molecular Mismatch Dictates Early Risk of De Novo Donor-Specific Antibody Development and Offers a Personalized Approach to Immunosuppression Management in Kidney Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/hla-dr-dq-molecular-mismatch-dictates-early-risk-of-de-novo-donor-specific-antibody-development-and-offers-a-personalized-approach-to-immunosuppression-management-in-kidney-transplantation/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress