High Grade Cytomegalovirus Viremia Events in Solid Organ Transplant Recipients: Description and Quality Improvement
1Dept. of Pharmacy, Cleveland Clinic, Cleveland, OH
2Dept. of Infectious Diseases, Cleveland Clinic, Cleveland, OH.
Meeting: 2018 American Transplant Congress
Abstract number: B296
Keywords: Cytomeglovirus, Monitoring
Session Information
Session Name: Poster Session B: Non-Organ Specific: Economics, Public Policy, Allocation, Ethics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: High grade cytomegalovirus viremia (HGCMVV) often results in symptomatic disease requiring IV antiviral therapy and is associated with CMV recurrence and resistance. We postulated that HGCMVV would be a quality metric for CMV prevention strategies.
Methods: Retrospective cohort including adult and pediatric transplant recipients at Cleveland Clinic Ohio and Florida with plasma CMV DNA >100,000 IU/mL from 9/2015-6/2017. Data collected: organ transplanted, donor/recipient (D/R) CMV IgG status, immunosuppressives, CMV monitoring and prophylaxis strategy, time to HGCMVV, CMV disease classification and resistance.
Results: In the study period, 26 (2%) HGCMVV events occurred in 1,236 solid organ transplant recipients, 19 (73%) of whom were CMV D+/R-. Event rates were 4.7% heart, 3.1% intestine, 2.2% kidney, 1.5% lung and 0.8% liver. Eleven (46%) patients received depleting induction agents. Average tacrolimus concentration was >10 ng/mL in 12 (48%) patients before HGCMVV. HGCMVV was symptomatic requiring hospital admission for IV therapy in 25 (96%) patients. Proven or possible ganciclovir resistance developed in 2 (8%) and 5 (19%), respectively. Median time to HGCMVV from transplant and end of prophylaxis was 229 days (IQR 138-365) and 66 days (IQR 43-171), respectively. Following completion of prophylaxis, 75% of HGCMVV occurred within 6 months. CMV DNA frequencies immediately prior to HGCMVV were monthly (38%), bi-weekly (12%), weekly (12%), irregular (30%), and none (8%). By increasing the frequency to every 1-2 weeks for 6 months after prophylaxis completion, the number needed to test (NNT) to prevent 1 case of HGCMVV was 13 for CMV D+/R- patients and 50 for all patients transplanted.
Conclusions: HGCMVV and its morbidity is uncommon but largely preventable in the first year post-transplant. Assuming an appropriate pre-emptive strategy, we determined that monitoring CMV every 1-2 weeks in the 6 months post-prophylaxis would prevent most HGCMVV and related hospital admissions. Based on the NNT, vigilant monitoring would be most advantageous in CMV D+/R- patients. 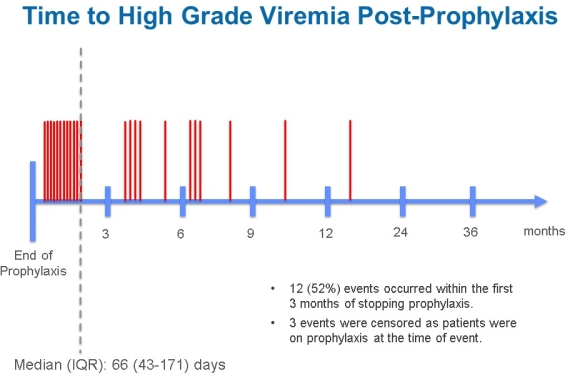
CITATION INFORMATION: Spinner M., Athans V., Koval C. High Grade Cytomegalovirus Viremia Events in Solid Organ Transplant Recipients: Description and Quality Improvement Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Spinner M, Athans V, Koval C. High Grade Cytomegalovirus Viremia Events in Solid Organ Transplant Recipients: Description and Quality Improvement [abstract]. https://atcmeetingabstracts.com/abstract/high-grade-cytomegalovirus-viremia-events-in-solid-organ-transplant-recipients-description-and-quality-improvement/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress
