Heterologous Vaccine Schedule Against Sars-CoV-2 with 2 Doses of Inactivated Virus and a Booster of mRNA BNT162b2 in Kidney Transplant Recipients
M. Seija1, F. Rammauro2, J. Santiago1, N. Orihuela3, C. Zulberti3, D. Machado4, C. Recalde4, J. Noboa1, R. Astesiano1, F. Yandian1, A. Guerisoli1, A. Morra3, D. Cassinelli1, A. Pippolo1, B. De Aramburu1, C. Coelho1, P. González1, R. Romero1, R. Rodriguez-Teja4, G. Acuña4, V. Rabaza4, N. Perg4, R. Cordero4, S. Orihuela3, L. Curi3, E. Burgstaller4, O. Noboa1, O. Pritsch2, M. Nin1, S. Bianchi5
1Centro de Nefrología, Hospital de Clínicas. Facultad de Medicina UdeLaR, Montevideo, Uruguay, 2Departamento de Inmunobiología, Facultad de Medicina, UdeLaR, Montevideo, Uruguay, 3Hospital Italiano, Montevideo, Uruguay, 4Hospital Evangélico, Montevideo, Uruguay, 5Departamento de Fisiopatología, Facultad de Medicina UdeLar, Montevideo, Uruguay
Meeting: 2022 American Transplant Congress
Abstract number: 978
Keywords: COVID-19, Kidney transplantation, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
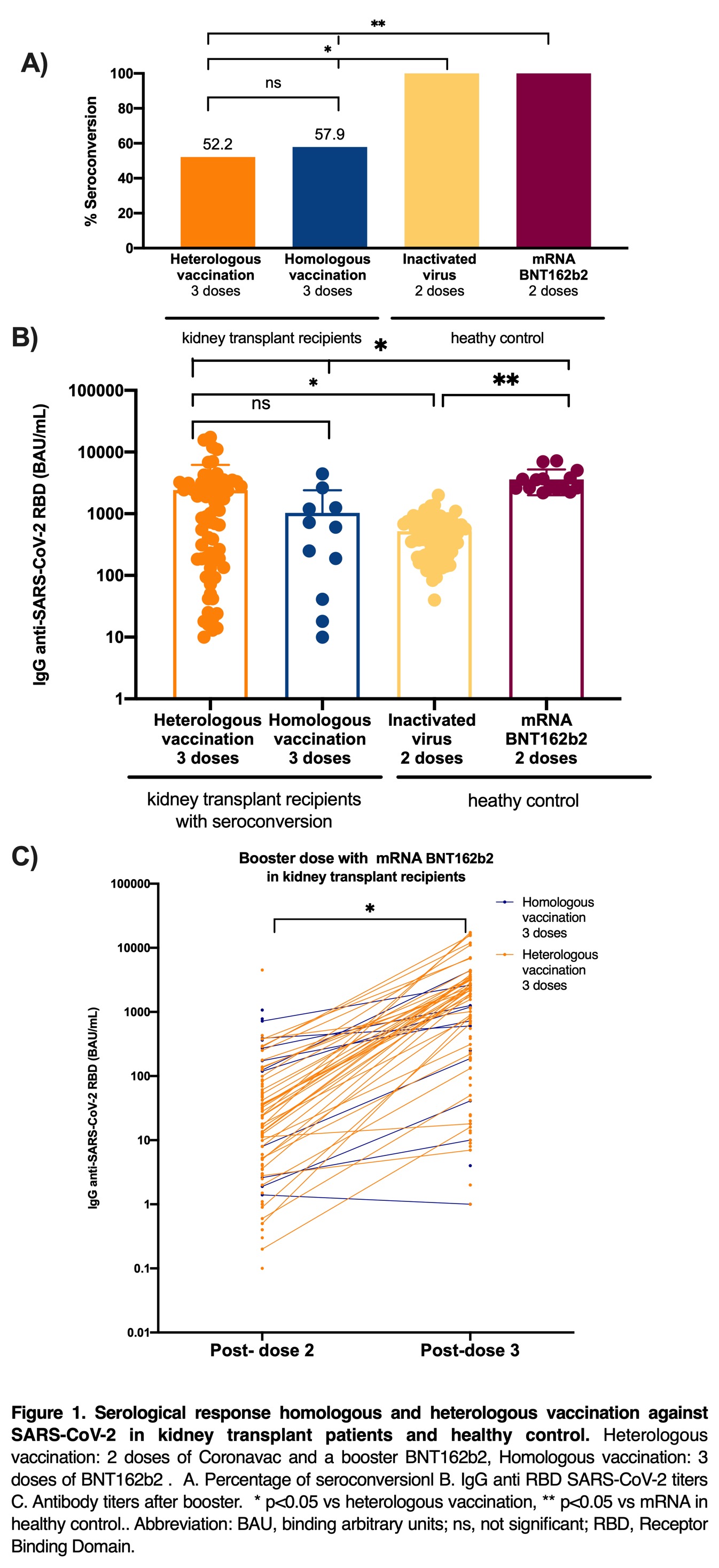
*Purpose: Emerging evidence suggests that 3 doses of SARS-CoV-2 mRNA vaccine enhance immunity in kidney transplant (KT) patients. However, few studies have focused on humoral response after inactivated virus-based vaccines. Here we report the results of humoral response in KT recipients in comparison with healthy control group after homologous and heterologous regimens with inactivated virus (Coronavac) and mRNA vaccine BNT162b2.
*Methods: A multicenter prospective study was conducted. KT recipients received heterologous vaccine schedule (2 doses of Coronavac and a booster of mRNA BNT162b2, n= 136) or homologous (3 doses of BNT162b2 n=19). Healthy control group received 2 doses or Coronavac (n=67) or BNT162b2 (n=15). Serum IgG antibodies against Receptor Binding Domain of SARS-CoV-2 Spike protein were determined 30 and 40 days after last dose.
*Results: Seroconversion was 52.2% and 57,9% with heterologous and homologous vaccination schedules in KT, p=0.789, figure 1. Among KT patients with seroconversion, antibody levels against RBD of SARS-CoV-2 were [1012 (183-3111) and 603 (41-1255) BAU/mL, with heterologous and homologous schedule, respectively. Levels were higher in KT compared to heathy control with 2 doses of inactivated virus 308 (209-335), p=0.03 and lower than heathy control with 2 doses of BNT162b2: 2638 (2608-3808) BAU/mL, p=0.001].
*Conclusions: Seroconversion improves after a third dose with homologous or heterologous vaccine schedules. Among patients with seroconversion antibody levels were higher than in heathy control with two doses of inactivated virus. Measurement of antibody levels could help to improve vaccination policies.
To cite this abstract in AMA style:
Seija M, Rammauro F, Santiago J, Orihuela N, Zulberti C, Machado D, Recalde C, Noboa J, Astesiano R, Yandian F, Guerisoli A, Morra A, Cassinelli D, Pippolo A, Aramburu BDe, Coelho C, González P, Romero R, Rodriguez-Teja R, Acuña G, Rabaza V, Perg N, Cordero R, Orihuela S, Curi L, Burgstaller E, Noboa O, Pritsch O, Nin M, Bianchi S. Heterologous Vaccine Schedule Against Sars-CoV-2 with 2 Doses of Inactivated Virus and a Booster of mRNA BNT162b2 in Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/heterologous-vaccine-schedule-against-sars-cov-2-with-2-doses-of-inactivated-virus-and-a-booster-of-mrna-bnt162b2-in-kidney-transplant-recipients/. Accessed February 20, 2026.« Back to 2022 American Transplant Congress