Ganciclovir Therapeutic Drug Monitoring in Obese Adults Patients – Opportunity for Clinical Utility
Y. Tran1, S. Bernard1, R. W. Stevens1, L. Myhre1, R. Razonable2
1Department of Pharmacy, Mayo Clinic, Rochester, MN, 2Division of Infectious Diseases, Department of Internal Medicine, Mayo Clinic, Rochester, MN
Meeting: 2021 American Transplant Congress
Abstract number: 773
Keywords: Cytomeglovirus, Ganciclovir, Infection, Obesity
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Ganciclovir (GCV) is the drug of choice for the treatment of cytomegalovirus (CMV) infection; optimal dosing of GCV in the obese patient population is unknown. The primary objective of this study is to assess the frequency of ganciclovir therapeutic drug monitoring (TDM) obtained in obese patients with prolonged use. Secondary objectives are to explore the role of GCV TDM and its associated clinical outcomes, understand barriers to clinical utilization of GCV TDM, and improve our institutional protocol.
*Methods: This was a retrospective, multicenter chart review spanning Jan 2014-Jun 2020. Patients were included if they were adults with obesity (BMI ≥ 40 kg/m2 and/or actual body weight ≥ 120 kg) and GCV therapy indicated for treatment of CMV viremia, CMV syndrome, suspected or biopsy-proven CMV invasive disease, or other viruses. A qualitative survey among clinical pharmacists was also completed to assess the perspective and practices with regards to GCV TDM.
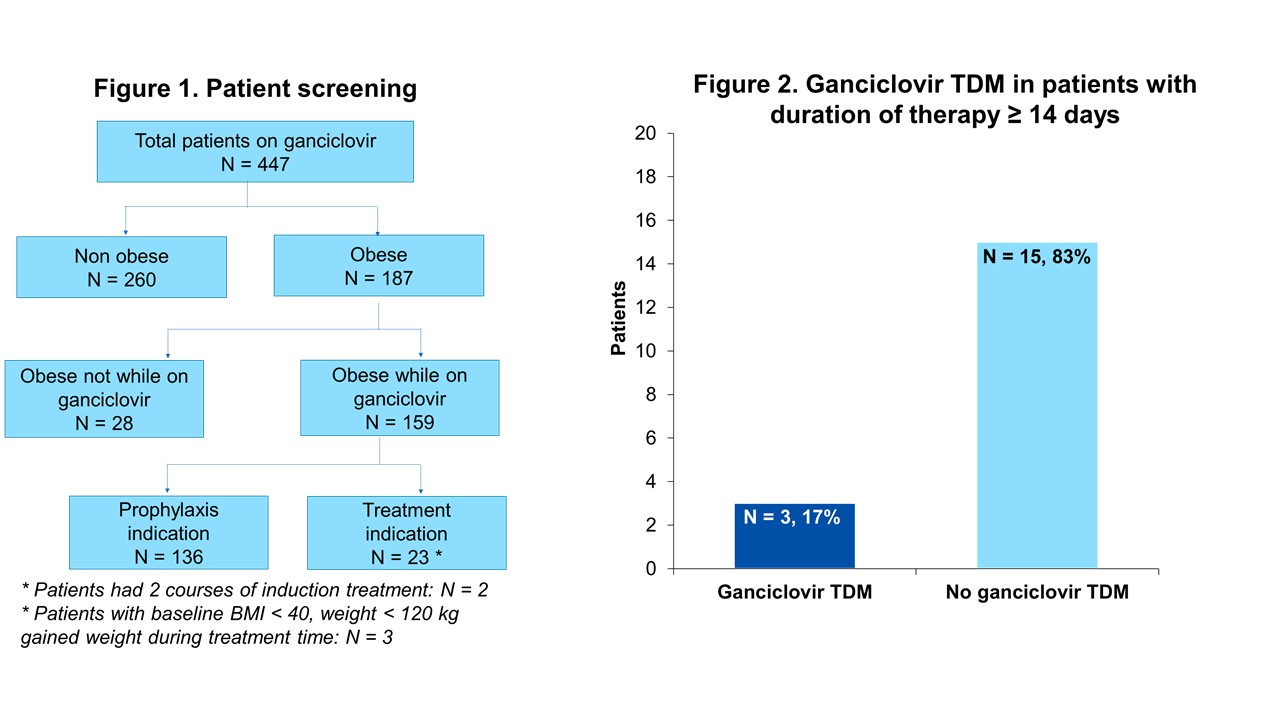
*Results: This study screened 447 patients receiving GCV. A total of 23 (5%) were obese, with 2 patients having 2 separate courses of induction (Figure 1). Baseline characteristics are in Table 1. The majority of episodes were indicated for the treatment of CMV viremia (n=9, 36%) or suspected CMV-tissue-invasive disease (n=7, 28%). Among the 18 patients with duration of therapy ≥ 14 days, GCV TDM was completed for 3 patients (17%) (Figure 2) and time from treatment initiation to TDM ranged from 21 to 18 days (Table 3). The pharmacist survey revealed support for use of GCV TDM; however, additional evidence was desired (Table 4).
*Conclusions: GCV TDM in obese patients is potentially underutilized at our institution. This is complicated by a limited number of obese patients contracting CMV. This topic requires further study with additional patients undergoing GCV TDM to explore safety and efficacy outcomes relevant to GCV TDM in the obese patient population.
To cite this abstract in AMA style:
Tran Y, Bernard S, Stevens RW, Myhre L, Razonable R. Ganciclovir Therapeutic Drug Monitoring in Obese Adults Patients – Opportunity for Clinical Utility [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/ganciclovir-therapeutic-drug-monitoring-in-obese-adults-patients-opportunity-for-clinical-utility/. Accessed March 9, 2026.« Back to 2021 American Transplant Congress