Five Year Survival and Graft Function with the Clinical Use of Gene Expression Profiling for Rejection Surveillance
Heart and Vascular Institute, University of Pittsburgh, Pittsburgh, PA
Pharmacy and Therapeutics, University of Pittsburgh, Pittsburgh, PA
Meeting: 2013 American Transplant Congress
Abstract number: 140
Purpose: Gene expression profiling (GEP) offers a non-invasive alternative to surveillance with endomyocardial biopsies. We sought to assess the medium-term survival and graft function in patients managed with and without GEP in general clinical use compared to low-risk controls.
Methods: All patients with CTX at a single institution from 1/02 until 9/11. GEP was typically started after one year, all patients who had at least one GEP test were included in the GEP group. The control group consisted of low-risk patients who survived at least one year post-transplant and had no significant rejection in the first year. Controls were transplanted before GEP was available, declined GEP use or were not considered for testing.
Results: A total of 283 patients were included, 151 in the GEP group and 132 controls. Baseline characteristics were not different between groups: mean age 55 years, 77% male, 89% white, 55% ischemic, VAD 25%, CMV mismatch 23%, 18% with PRA > 20. Donor characteristics were not different: mean age 35 years, 77% white and ischemic time 190 minutes. The control group had fewer male donors 60% v. 72%, p=0.025, Induction use was more common in controls 77% (80% alemtuzumab) v. GEP 66% (73% alemtuzumab), p=0.044. Maintenance was not different for groups: 99% TAC, 99%MMF. The median time to first GEP was 496 days (range 143 to 1440), with a median number of GEP assessments of 3 (range 1-9). The 5 year survival was significantly higher for GEP group, 90% v. 78%, p=0.01. The 5 year freedom from EF < 50% was also significantly better in the GEP group, 92% v. 80%, p=0.01. Only one patient in each group died of graft failure after one year.
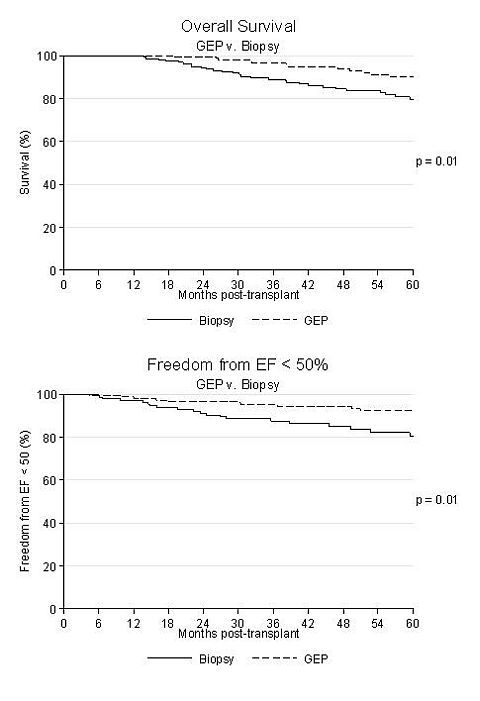
Conclusions: Medium-term survival and graft function were better in patients who had GEP for rejection surveillance than low-risk controls who had at least one year of rejection-free survival.
To cite this abstract in AMA style:
Teuteberg J, Yost C, Newman C, Grabowski C, Kormos R, McNamara D, Zomak R, Bermudez C, Shullo M. Five Year Survival and Graft Function with the Clinical Use of Gene Expression Profiling for Rejection Surveillance [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/five-year-survival-and-graft-function-with-the-clinical-use-of-gene-expression-profiling-for-rejection-surveillance/. Accessed June 30, 2025.« Back to 2013 American Transplant Congress
