Five Year Outcomes with Alemtuzumab Induction Therapy in Elderly Renal Transplant Recipients.
Pharmacy, Northwestern Memorial Hospital, Chicago, IL
Meeting: 2017 American Transplant Congress
Abstract number: 423
Keywords: Induction therapy, Kidney transplantation, Safety, Survival
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Induction Therapy
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:30pm-3:42pm
Presentation Time: 3:30pm-3:42pm
Location: E354b
Background:
The elderly population (≥ 65 years) comprise one fourth of patients on the waiting list for renal transplantation. Alemtuzumab use has increased over the past decade in the United States and is the induction agent of choice for most renal transplant recipients at Northwestern Memorial Hospital (NMH). Use of alemtuzumab in the elderly has been associated with an increased risk of transplant related complications and thus has been avoided in patients 65 years and older at NMH since October 2014.
Purpose:
To compare 5-year outcomes of patient and allograft survival between renal transplant recipients who are ≥ 65 years and those <65 years who received alemtuzumab induction.
Methods:
This is a single center, retrospective cohort study of patients who received alemtuzumab induction for renal transplantation. Patients 65 years and older who were transplanted between January 1st 2007 and October 31st 2009 were included. These patients were matched 1:1 with patients less than 65 years of age during the same time period by induction agent, donor type (living or deceased; SCD, DCD or ECD), and transplant year. Patient and allograft survival at 1, 3 and 5 years post-transplant were examined.
Results:
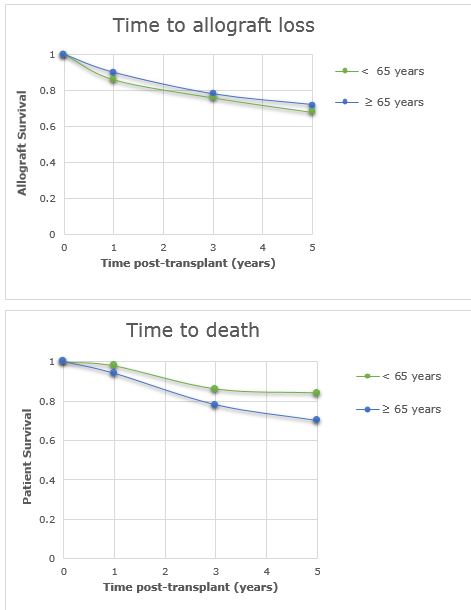
A total of 100 patients (50 patients included in both ≥ 65 years and <65 years) were included in the study. Patient demographics are summarized in Figure 1. Patient survival at 1 (p=0.6098), 3 (p=0.4349), and 5 years (0.1539) were similar between groups. There was no difference in graft survival data at 1 (p=0.7583), 3 (p=0.8122), and 5 years (p=0.8273) (Figure 2).
Conclusions:
Alemtuzumab use in elderly renal transplant recipients was not associated with worse patient or allograft survival up to 5 years post-transplant when compared with a matched cohort.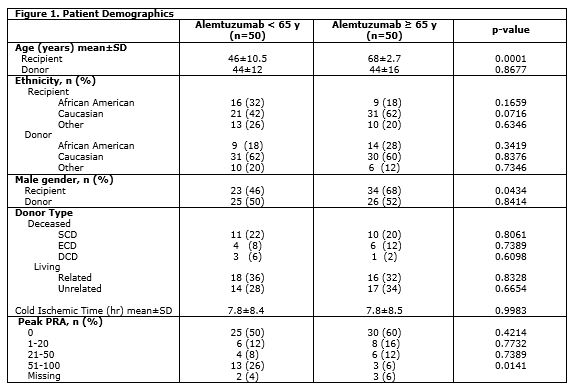
CITATION INFORMATION: Casagrande M, Cunningham K, Tseng C, D'Agostino C, Wong L, Richardson C. Five Year Outcomes with Alemtuzumab Induction Therapy in Elderly Renal Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Casagrande M, Cunningham K, Tseng C, D'Agostino C, Wong L, Richardson C. Five Year Outcomes with Alemtuzumab Induction Therapy in Elderly Renal Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/five-year-outcomes-with-alemtuzumab-induction-therapy-in-elderly-renal-transplant-recipients/. Accessed February 17, 2026.« Back to 2017 American Transplant Congress
