Final Results from the BENEFIT-EXT Trial: A 7 Year Follow-Up of Belatacept Treated Patients
1Mt Sinai Med Ctr, NY
2Hosp do Rim e Hipertensão, Sao Paulo, Brazil
3Inst de Nefrologia, Buenos Aires, Argentina
4Univ Hospital, Toulouse, France
5Univ Hospital Leuven, Leuven, Belgium
6Vienna Transplantation Ctr, Vienna, Austria
7BMS, Lawrenceville
8Univ Hosp Bellvitge, Barcelona, Spain
9Univ Hosp of Bicêtre, Villejuif, France.
Meeting: 2015 American Transplant Congress
Abstract number: 306
Keywords: Graft survival, Immunosuppression, Kidney transplantation, Renal function
Session Information
Session Name: Concurrent Session: Kidney: Novel Agents
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 4:24pm-4:36pm
Presentation Time: 4:24pm-4:36pm
Location: Terrace I-III
Background: At 3 and 5yrs post-transplant, BENEFIT-EXT showed a renal function benefit and consistent safety in belatacept (bela) treated pts vs cyclosporine (CsA). Final 7 yr results for all randomized pts in BENEFIT-EXT are reported herein.
Methods: Recipients of extended criteria donor kidneys were randomized to more (MI) or less (LI) intensive bela or CsA. 7 yr outcomes were assessed among all randomized and transplanted pts. In a prospective, post hoc, exploratory analysis, time to death or graft loss was compared between treatment groups using a Cox regression analysis. HR estimates and 95% CIs are provided.
Results: BENEFIT-EXT included 184 bela MI, 175 bela LI and 184 CsA pts; 74 MI, 84 LI and 57 CsA pts completed 7 yrs. Over 7 yrs, HR estimates comparing time to death/graft loss were 0.932 for MI vs CsA and 0.944 for LI vs CsA (Fig). Mean cGFR (MDRD) was 57.6 (MI), 59.1 (LI) and 44.6 (CsA) mL/min/1.73m2. Rate of freedom from death/graft loss/cGFR <30 mL/min/1.73m2 was 53% MI, 55% LI and 36% CsA. Acute rejection occurred in 19% of pts in both bela (MI and LI) groups and 16% of the CsA group. SAEs occurred in 87% MI, 89% LI and 84% CsA pts. Across MI, LI and CsA, respectively, incidence per 100 person-yrs of serious infections (21.7, 15.8, 19.6); viral infections (21.0, 17.5, 19.1); fungal infections (9.8, 6.9, 11.0) and malignancies (3.7, 3.1, 3.4), were similar. 10 PTLD cases were observed: 2 MI (1 EBV+ [per 100 person-yrs, 0.11] and 1 EBV- [1.61]); 7 LI (2 EBV+ [0.23] and 5 EBV- [6.10]); and 1 CsA (EBV+ [0.13]).
Conclusions: At 7-yrs post-transplant, bela was associated with sustained improvement in renal function vs CsA. The bela safety profile was consistent with past reports. 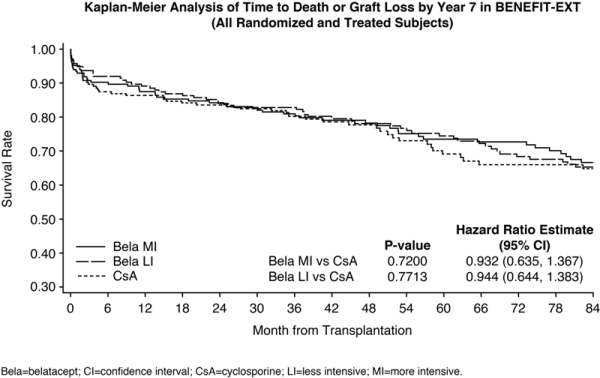
To cite this abstract in AMA style:
Florman S, Pestana JMedina, Rial M, Rostaing L, Kuypers D, Mühlbacher F, Polinsky M, Meier-Kriesche U, Grinyó J, Durrbach A. Final Results from the BENEFIT-EXT Trial: A 7 Year Follow-Up of Belatacept Treated Patients [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/final-results-from-the-benefit-ext-trial-a-7-year-follow-up-of-belatacept-treated-patients/. Accessed March 9, 2026.« Back to 2015 American Transplant Congress
