Extended Duration of Rabbit Antithymocyte Globulin (rATG) Induction in Kidney and Kidney/Pancreas Transplant Recipients
Temple University Hospital, Philadelphia, PA
Meeting: 2022 American Transplant Congress
Abstract number: 1376
Keywords: Economics, Induction therapy, Kidney/pancreas transplantation
Topic: Clinical Science » Kidney » 37 - Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Many transplant centers have endorsed an expanded duration of rabbit antithymocyte globulin (rATG) in the outpatient setting through peripheral administration to help minimize cost. To reduce cost and hospital length of stay (LOS), Temple University Hospital (TUH) implemented a protocol to administer rATG every 48 hours to maximize the number of infusions administered in the outpatient setting. The aim of this study was to evaluate the utilization of rATG in kidney and kidney/pancreas recipients in the inpatient and outpatient setting as well as safety and efficacy in patients receiving extended duration of therapy.
*Methods: This was a retrospective, single-center, cohort study of adult kidney and kidney/pancreas transplant recipients between 08/18/2016 – 04/30/2020. All patients who received a kidney or simultaneous kidney/pancreas transplant at TUH were included. Patients who received alemtuzumab or basiliximab for induction were excluded. The primary endpoint was to assess the total cost of rATG. Secondary endpoints included total cumulative rATG dose, biopsy proven acute rejection (BPAR), time to completion of rATG, graft loss within 1 month and adverse events such as bone marrow suppression and infusion related reactions.
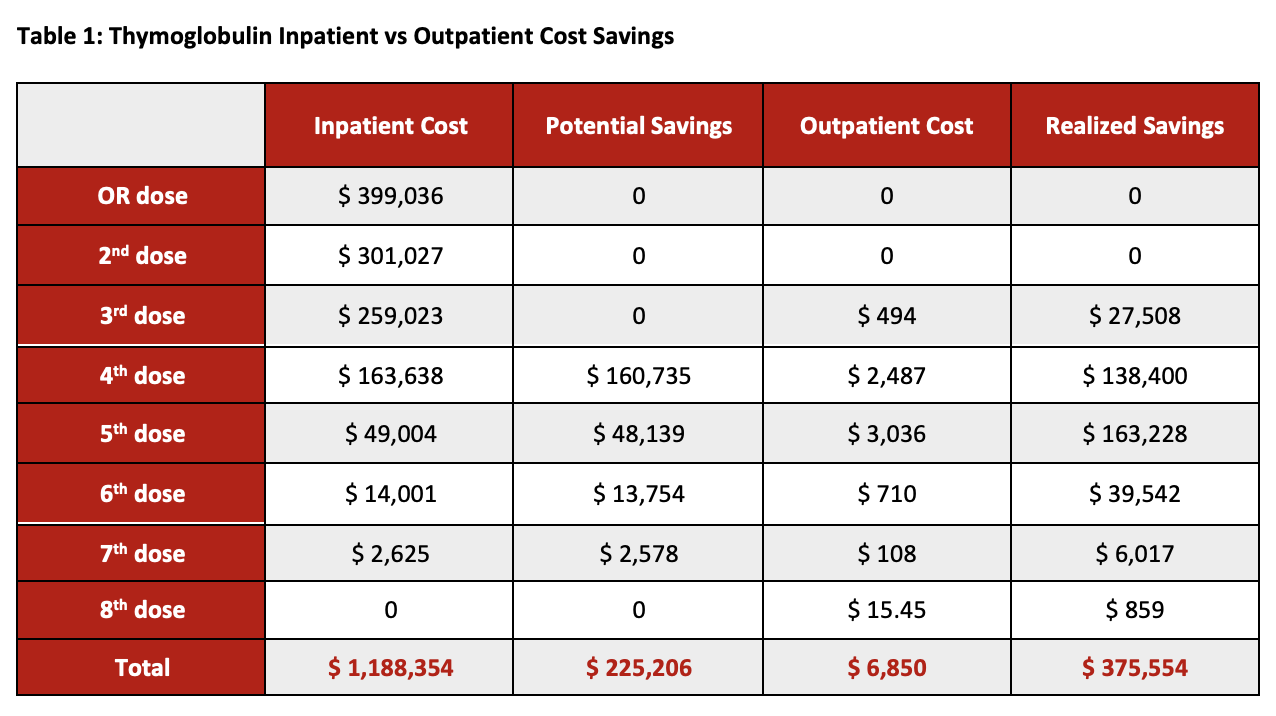
*Results: A total of 110 patients were included in the analysis. Mean age was 52 years and 74% of patients were male. The majority of patients received a deceased donor transplant (86%) and 39% were African American. The mean total weight-based rATG dose was 4.7 mg/kg and 43% of patients were deemed high immunologic risk. The mean hospital LOS was 5 days and 99% of patients received at least four doses of rATG. The mean time to rATG completion was 8 days. A low number of patients experienced BPAR (6%) and no graft loss was identified. Readmissions within 1 month occurred in 32% of patients. rATG was well tolerated with no adverse events noted. A realized cost savings of $375,554 was identified after adoption of the every 48 hour dosing protocol. The total outpatient cost of rATG was $6,850 for all patients. Furthermore, the total inpatient cost was $1,188,354 which could have been reduced by $255,206 if the last inpatient dose was administered in the outpatient setting. Further cost information is available in Table 1.
*Conclusions: After implementation of an every 48 hour rATG dosing protocol, this demonstrated an enhanced cost savings without compromising graft outcomes or increasing risk of adverse events.
To cite this abstract in AMA style:
Majmundar D, Carlo ADi, Diamond A. Extended Duration of Rabbit Antithymocyte Globulin (rATG) Induction in Kidney and Kidney/Pancreas Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/extended-duration-of-rabbit-antithymocyte-globulin-ratg-induction-in-kidney-and-kidney-pancreas-transplant-recipients/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress