Everolimus With Low Dose Tacrolimus in Intestinal Transplantation
Miami Transplant Institute, University of Miami Miller School of Medicine, Miami, FL.
Meeting: 2015 American Transplant Congress
Abstract number: A288
Keywords: Immunosuppression, Intestinal transplantation, Rejection, Renal dysfunction
Session Information
Session Name: Poster Session A: Small Bowel All Topics
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Introduction: Renal dysfunction is a well-known complication after organ transplant given immunosuppression (ISP) with calcineurin inhibitors. This is especially true after intestinal transplant (ITx) given the need for increased ISP. The combination of mTOR-inhibitors with tacrolimus (TAC) is common practice to decrease nephrotoxicity. Despite reports describing the use of sirolimus in intestinal transplant, we are not aware of previous experience with everolimus (EVE). Everolimus has a safer side effect profile than sirolimus and its anti-proliferative properties may play a role in preventing late graft dysfunction due to chronic rejection in ITx.
Methods: We evaluated patients who underwent an ITx and received everolimus in combination with tacrolimus for a minimum of 1 year.
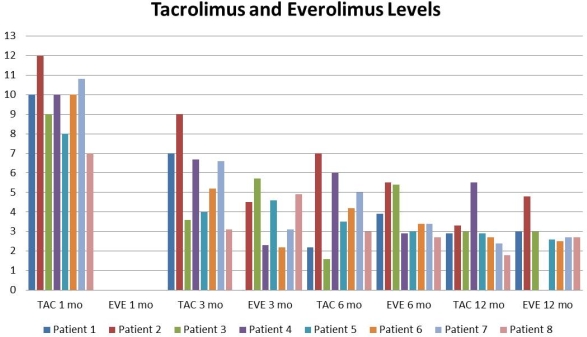
Results: 8 patients met inclusion criteria. The average age was 47 years (24-60). TAC was used as maintenance ISP. EVE was introduced after 30 days post-transplant. All patients are steroid free. If the patient presented with any healing problem, EVE was held. Average time for initiation of EVE was 1.6 months (1-3). Creatinine is shown in Figure 1, TAC and EVE levels are shown in Figure 2.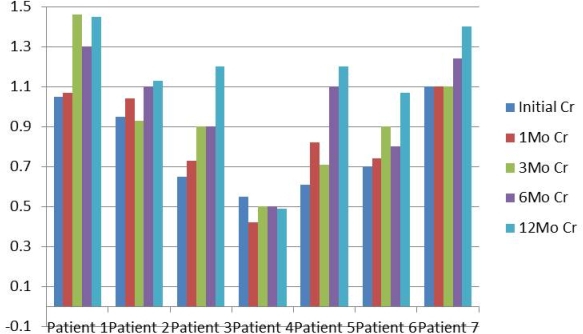
 No serious adverse effects of EVE were noted. 2 patients presented with oral ulcers and 2 patients presented with minor leg edema. 2 patients had rejection before the introduction of EVE. There were no patients with rejection after EVE was started. No CMV infection or PTLD was noted. Survival was 100% for this group.
No serious adverse effects of EVE were noted. 2 patients presented with oral ulcers and 2 patients presented with minor leg edema. 2 patients had rejection before the introduction of EVE. There were no patients with rejection after EVE was started. No CMV infection or PTLD was noted. Survival was 100% for this group.
Conclusion: Low dose TAC in combination with EVE seems to be a safe and excellent alternative to tacrolimus monotherapy. Renal function remains stable after one year post-transplant. Longer follow-up is required to determine the effect of everolimus on late graft dysfunction.
To cite this abstract in AMA style:
Beduschi T, Garcia J, Lugo-Baruqui J, Fan J, Nishida S, Jebrock J, Tekin A, Selvaggi G, Ruiz P, Vianna R. Everolimus With Low Dose Tacrolimus in Intestinal Transplantation [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/everolimus-with-low-dose-tacrolimus-in-intestinal-transplantation/. Accessed March 7, 2026.« Back to 2015 American Transplant Congress
