Evaluation of the Safety and Tolerability of Clazakizumab® (Anti-IL-6 Monoclonal) as a Desensitization Agent in Highly-HLA Sensitized ESRD Patients (nct03380962)
Cedars Sinai Medical Ctr, Los Angeles, CA
Meeting: 2019 American Transplant Congress
Abstract number: 27
Keywords: Highly-sensitized, HLA antibodies, Kidney transplantation, Plasmapheresis
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Desensitization
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:42pm-2:54pm
Presentation Time: 2:42pm-2:54pm
Location: Ballroom A
*Purpose: Currently, there is growing interest in development of novel immune-modulatory drugs likely to improve allo-antibody reduction in transplantation. Clazakizumab (Vitaeris Inc.) is a humanized monoclonal antibody aimed at the cytokine IL-6. Here we undertook a single center Phase I/II open label pilot study to assess safety and efficacy of clazakizumab as a desensitization agent in highly-HLA sensitized (HS) patients awaiting HLAi renal transplantation.
*Methods: From March 2018 to present, 9 HS patients (cPRA >50%) received plasma exchange (PLEX) x5 sessions followed by IVIg 2gm/kg (maximum 140 grams) X 1. One week post-IVIg, the patients received clazakizumab, 25mg SC Q4W X 6 doses with monitoring of HLA antibody levels and other immune parameters. Study end point was transplantation by day 270 post-Rx. If transplanted, patients received 6 additional doses of clazakizumab, 25mg SC Q4W starting 5-7 days post-transplant, with a 6-month protocol biopsy. The primary end point of this open label study was reduction of HLA antibodies from baseline. All transplanted HS patients received antibody induction with alemtuzumab and were maintained on standard immunosuppression with tacrolimus, cellcept, and tapering prednisone.
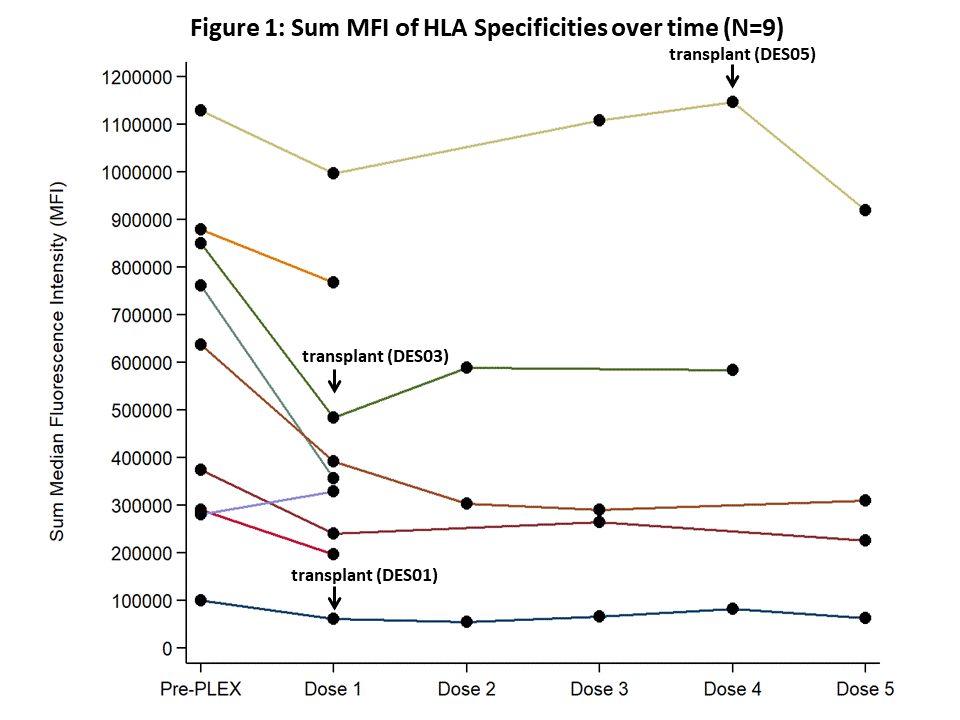
*Results: Nine patients have been enrolled to date. Sum MFI of HLA specificities over time for individual study patient is shown {Fig 1}. Specificities ranged from 100,000-1,100,000 MFI pre-PLEX and was reduced to 0-950,000 MFI by dose #5 of clazakizumab. Previously, we noted that anti-HLA antibodies tended to rebound by approximately 1-3 months after completion of PLEX/IVIg. Here, with monthly clazakizumab, MFI values remained stable and did not rebound when compared to pre-PLEX. Four transplants were performed to date. Three of 4 patients had FCMX+ and DSA+ at time of transplant. One graft was lost to a technical issue at transplant. The patient was continued on clazakizumab post-nephrectomy with no HLA antibody rebound and with subsequent successful transplant 7 months later. SAEs included wound dehiscence requiring wound re-closure (1), hematuria and UTI (1), and bacteremia prior to receiving 1st dose of study drug (1), all felt unrelated to clazakizumab.
*Conclusions: Although preliminary, clazakizumab appears promising as a potential desensitization agent when used with PLEX + IVIg. Reducing the risk of antibody rebound post-PLEX + IVIg may aid in achieving successful HLAi transplantation for these patients.
To cite this abstract in AMA style:
Vo AA, Ammerman N, Huang E, Peng A, Najjar R, Sethi S, Williamsons S, Myers C, Lim K, Choi J, Jordan SC. Evaluation of the Safety and Tolerability of Clazakizumab® (Anti-IL-6 Monoclonal) as a Desensitization Agent in Highly-HLA Sensitized ESRD Patients (nct03380962) [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-the-safety-and-tolerability-of-clazakizumab-anti-il-6-monoclonal-as-a-desensitization-agent-in-highly-hla-sensitized-esrd-patients-nct03380962/. Accessed March 1, 2026.« Back to 2019 American Transplant Congress