Evaluation of Empiric Extended-Release Tacrolimus Dosing in Adult Kidney Transplant Recipients
Pharmacy, NewYork-Presbyterian Hospital, New York, NY
Meeting: 2021 American Transplant Congress
Abstract number: 940
Keywords: Immunosuppression, Kidney transplantation, Obesity, Pharmacokinetics
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Envarsus XR® (extended-release [XR] tacrolimus) is the standard of care tacrolimus formulation at our center for de novo maintenance immunosuppression in adult kidney transplant recipients (KTR). At our center, KTR empirically begin XR tacrolimus at a dose of 0.12 mg/kg/day. The purpose of this study is to evaluate weight-based dosing requirements at various times post-transplant.
*Methods: This was an IRB-approved, single center retrospective analysis of adult patients who received a KT between 07/2019-07/2020. Patients were excluded if they received formulations other than XR tacrolimus or received medications known to interact with tacrolimus within the first two weeks following KT. The primary endpoint was the weight-based XR tacrolimus dose at first therapeutic tacrolimus trough level, defined as at least 2 consecutive trough levels between 8-12 ng/mL. Secondary endpoints included weight-based XR tacrolimus dose at postoperative days (POD) 7 and 14, and time to therapeutic tacrolimus trough level. Additional sub-group stratifications for sex, race, and weight were evaluated.
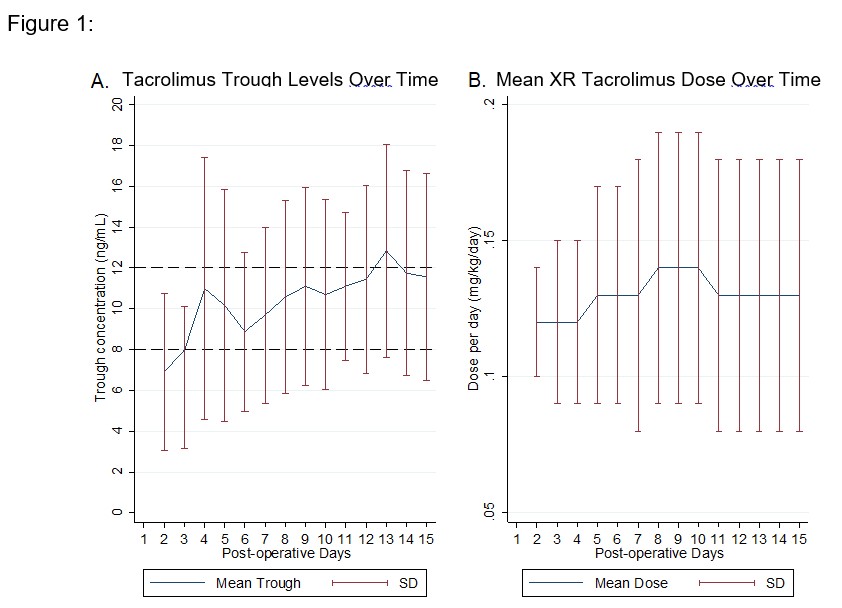
*Results: A total of 141 KTR were identified. Median patient age was 57 (46-64) years. Patients were predominantly male (60%) and white (45%). Median BMI was 26.9 (22.3-30.5) kg/m2. A total of 67 patients achieved a therapeutic tacrolimus trough level within the first 14 days following KT. Median weight-based XR tacrolimus dose at first therapeutic tacrolimus trough level was 0.12 (0.11-0.14) mg/kg/day and the median time to therapeutic trough level was 5 (4-9) days (Figure 1A and B). Median weight-based therapeutic XR tacrolimus doses on PODs 7 and 14 were 0.13 (0.1-0.17) and 0.13 (0.09-0.17) mg/kg/day, respectively. Median therapeutic weight-based XR tacrolimus dose for male patients was 0.13 (0.11-0.17) versus 0.12 (0.1-0.13) mg/kg/day for female patients (p=0.033). In patients with a BMI of <24, 24-29, and >29 kg/ m2, the median therapeutic weight-based XR tacrolimus dose was 0.14 (0.12-0.17), 0.11 (0.1-0.14), and 0.12 (0.1-0.14) mg/kg/day, respectively (p=0.022). The therapeutic dose was 0.12 (0.1-0.15) for patients who were Caucasian, African American, Asian, and other ethnicities and 0.13 (0.12-0.18) for Hispanic patients (p=0.796).
*Conclusions: XR tacrolimus starting doses of 0.12 mg/kg/day allowed for attainment of goal tacrolimus trough blood levels in most patients following KT. Accounting for patient gender and BMI when dosing XR tacrolimus may permit earlier achievement of therapeutic trough blood levels, however additional study is required to further evaluate this.
To cite this abstract in AMA style:
Patel C, Lee S, Salerno DM, Lange NW, Hedvat J. Evaluation of Empiric Extended-Release Tacrolimus Dosing in Adult Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-empiric-extended-release-tacrolimus-dosing-in-adult-kidney-transplant-recipients/. Accessed July 3, 2025.« Back to 2021 American Transplant Congress