Evaluation According to Donor Source: 24-Month Results from Post-Hoc Analysis of the ELEVATE Study in Kidney Transplant Recipients.
ELEVATE study group, Leiden, Netherlands.
Meeting: 2016 American Transplant Congress
Abstract number: B113
Keywords: Efficacy, Kidney transplantation, Renal function
Session Information
Session Name: Poster Session B: Drug Minimization
Session Type: Poster Session
Date: Sunday, June 12, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background: Several studies reported that recipients receiving renal allograft from living donors have better efficacy outcomes than the deceased donor counterparts. This post-hoc analysis of ELEVATE study evaluated the 24 month (M) efficacy and safety based on donor category (living vs deceased), comparing early conversion to everolimus (EVR) vs standard calcineurin inhibitor (CNI) in renal transplant recipients (RTxRs).
Methods: ELEVATE (NCT01114529) is a 24M, multicenter, open-label trial randomized de novo RTxRs 10–14 weeks post-Tx to convert from CNI to EVR (n = 360; C0 6–10 ng/mL) or to continue CNI (n = 357; C0: TAC 5–10 ng/mL [n=231], CsA 100–250 ng/mL [n = 126]); all received enteric-coated mycophenolate sodium and steroids. Composite efficacy endpoint (treated biopsy-proven acute rejection; tBPAR [Banff ≥IB], graft loss, or death); renal function (eGFR) and safety at M24 were analyzed by donor type (living vs deceased).
Results: At M24, the incidence of composite efficacy failure (CEF) and its components was lower in recipients of living vs deceased donor for both arms. Recipients of living vs deceased donor also had better mean eGFR across the treatment arms. Overall mean eGFR for recipients of living and deceased donor was higher in EVR vs CNI arms. Overall, safety was comparable among both the donor sources of EVR and CNI groups (Table).
Conclusion: Regardless of the treatment arm, recipients of living kidney donors had lower incidence of CEF and better renal function compared to deceased donors. Safety was comparable between recipients of living and deceased donors.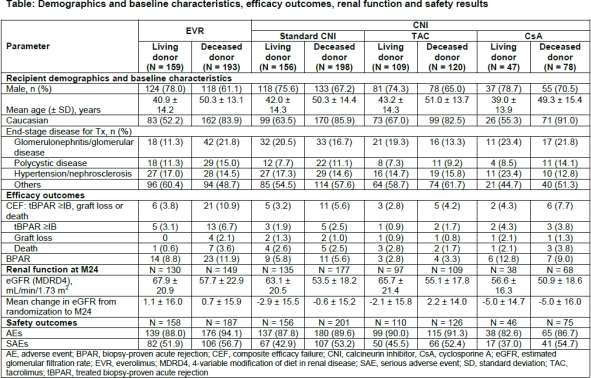
CITATION INFORMATION: de Fijter J, Holdaas H, Lopez P, Bernhardt P, Gaohong D, Cruzado J, van der Giet M. Evaluation According to Donor Source: 24-Month Results from Post-Hoc Analysis of the ELEVATE Study in Kidney Transplant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Fijter Jde, Holdaas H, Lopez P, Bernhardt P, Gaohong D, Cruzado J, Giet Mvander. Evaluation According to Donor Source: 24-Month Results from Post-Hoc Analysis of the ELEVATE Study in Kidney Transplant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-according-to-donor-source-24-month-results-from-post-hoc-analysis-of-the-elevate-study-in-kidney-transplant-recipients/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
