Evaluate the Effect of Cresemba (isavuconazonium Sulfate) Capsule and Noxafil (posaconazole) Delayed Release Tablets on Tacrolimus Dose to Concentration (D/C) Ratios in Lung Transplant Recipients
H. Sweiss1, E. Kincaide1, D. Levine2, R. Hall1
1University Health, San Antonio, Department of Pharmacotherapy and Pharmacy Services, The University of Texas at Austin, Pharmacotherapy Division, College of Pharmacy, San Antonio, TX, 2University Health, San Antonio, University of Texas Health Science Center at San Antonio, San Antonio, TX
Meeting: 2021 American Transplant Congress
Abstract number: 153
Keywords: Drug interaction, Fungal infection, Lung, Pharmacokinetics
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: The Metabolism Milleu: Updates in Pharmacokinetics and Pharmacogenomics
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 6, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:15pm-6:20pm
Presentation Time: 6:15pm-6:20pm
Location: Virtual
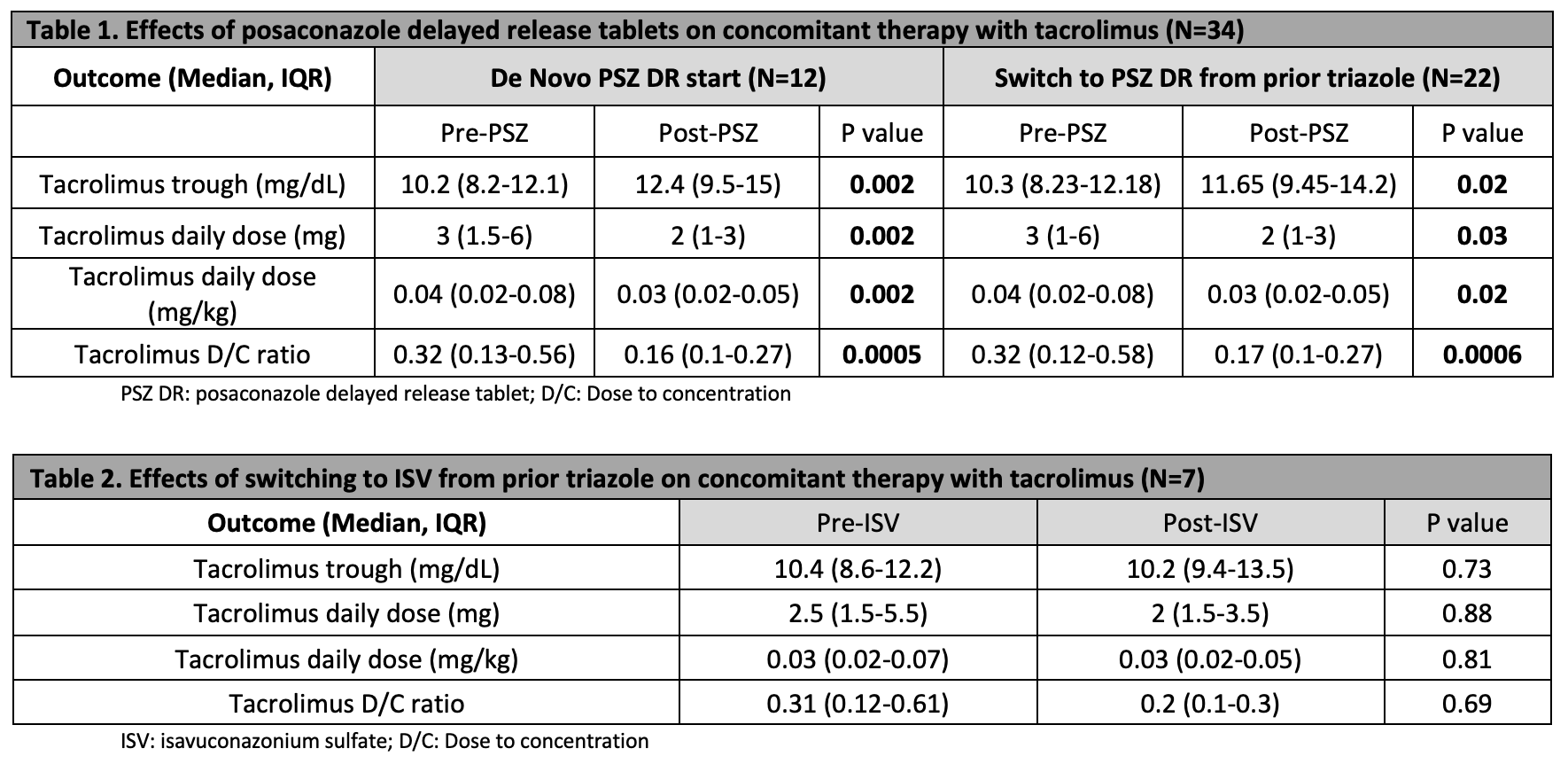
*Purpose: Evaluate the effect of Cresemba (isavuconazonium sulfate) capsule and Noxafil (posaconazole) delayed release tablet on tacrolimus dose to concentration (D/C) ratio in lung transplant.
*Methods: This retrospective review included adult lung transplant recipients at University Transplant Center from 1/1/2017-10/1/2020. Patients received concomitant therapy of immediate-release tacrolimus with one of the following triazoles for antifungal prophylaxis or treatment of invasive fungal disease for a minimum of 7 days: posaconazole (PSZ) delayed release (DR) tablet or isavuconazonium sulfate (ISV) capsule. A matched pair analysis compared the following outcomes pre-triazole initiation and 7-30 days post-triazole initiation: tacrolimus trough (mg/dL), total daily dose of tacrolimus (mg), total weight-based total daily dose of tacrolimus (mg/kg), and tacrolimus D/C ratio. In addition, percentage change in tacrolimus D/C ratio was calculated pre- and post-triazole initiation.
*Results: Fifty lung transplant recipients were screened for inclusion in the study, with 41 patients meeting study criteria. A total of 34/41 patients received PSZ DR tablets (Table 1). Of these patients, 22/34 were transitioned from previous triazole therapy to PSZ DR tablets and experienced a 47% reduction in tacrolimus D/C ratio after conversion to PSZ DR tablets. Twelve patients were newly initiated (de novo initiation) on PSZ DR tablets and experienced a 50% reduction in tacrolimus D/C ratio post-triazole initiation. A 35% reduction in tacrolimus D/C ratio was observed when transitioning to ISV from previous triazole therapy (P=0.69) (Table 2).
*Conclusions: This data suggests when initiating patients on PSZ DR tablets or converting from a previous triazole, a tacrolimus dose reduction of approximately 50% is required. Although there was not statistical significance, switching from a previous triazole to ISV may require less of a tacrolimus dose reduction. More patients would need to be gathered to see if this holds true. Limited evidence exists to navigate tacrolimus dose adjustments with de novo initiation and converting between triazole antifungals. This study provides guidance on management of tacrolimus dosing when initiating newer triazole antifungal agents.
To cite this abstract in AMA style:
Sweiss H, Kincaide E, Levine D, Hall R. Evaluate the Effect of Cresemba (isavuconazonium Sulfate) Capsule and Noxafil (posaconazole) Delayed Release Tablets on Tacrolimus Dose to Concentration (D/C) Ratios in Lung Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluate-the-effect-of-cresemba-isavuconazonium-sulfate-capsule-and-noxafil-posaconazole-delayed-release-tablets-on-tacrolimus-dose-to-concentration-d-c-ratios-in-lung-transplant-recipients/. Accessed March 9, 2026.« Back to 2021 American Transplant Congress