Efficacious Home Processing of Urine for Messenger RNA Profiling: A Point-of-Care Approach for Molecular Monitoring of Kidney Transplant Recipients
1WCM, NYC, NY, 2WCM, New York, NY
Meeting: 2019 American Transplant Congress
Abstract number: C128
Keywords: Gene expression, Kidney transplantation, Monitoring
Session Information
Session Name: Poster Session C: Kidney Technical
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Urinary mRNA profiling has been validated in multi-center studies as a robust tool for monitoring kidney transplant recipients. Home processing of urine samples for mRNA profiling by the kidney recipients themselves will reduce the logistic challenges associated with frequent in-center visits, allow for better monitoring, and help personalize care of kidney transplant recipients.
*Methods: In 3 sequential steps, we investigated 3 protocols towards developing a robust methodology for in-home processing of urine samples.
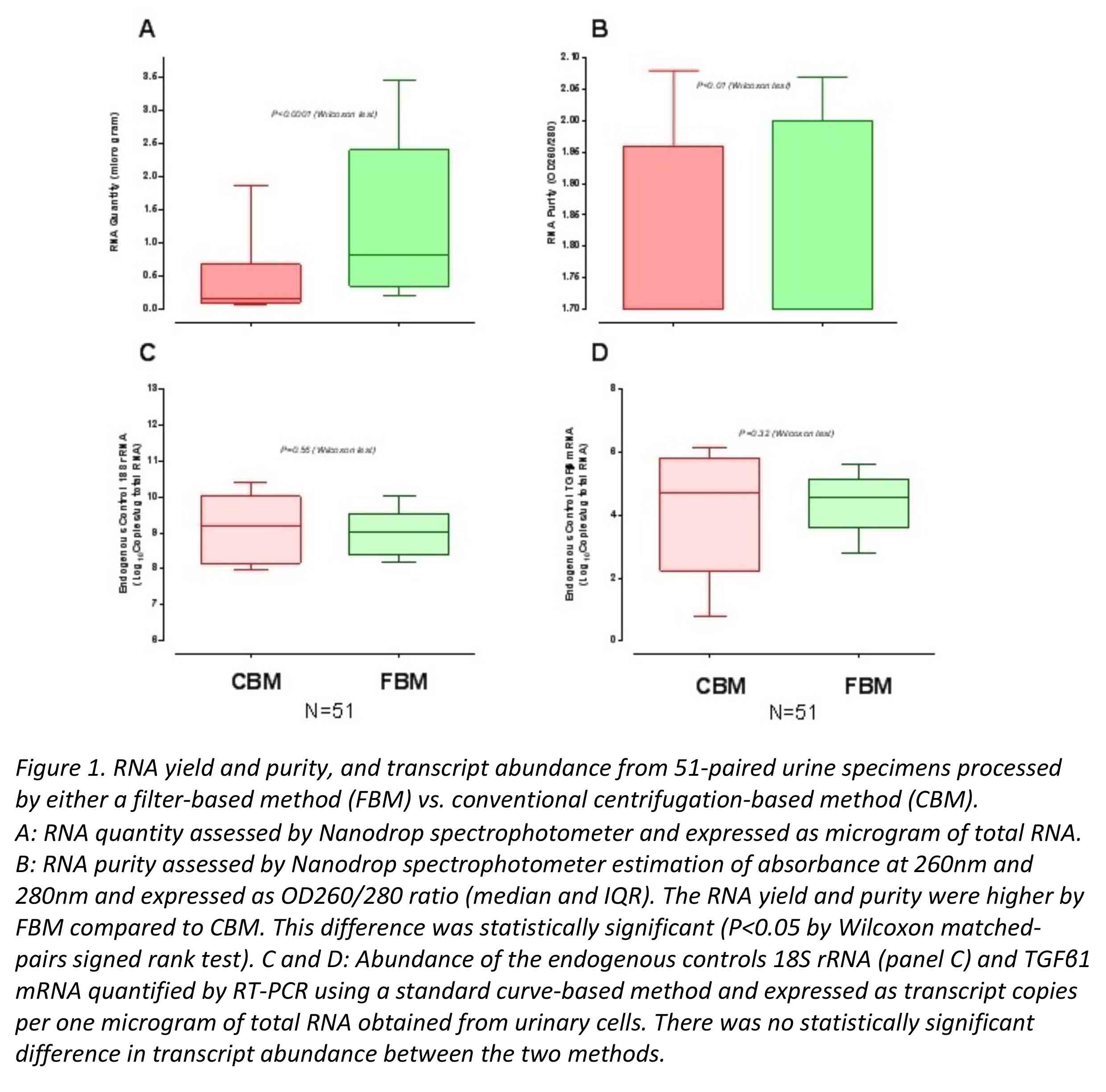
#1: We determined whether a filter-based method (FBM) is non-inferior to the conventional centrifugation-based method (CBM) with respect to RNA yield, purity, and transcript abundance (mRNA efficacy parameters [EF]). Freshly collected, 51 paired urine samples from 43 kidney recipients were processed using FBM or CBM by our laboratory staff and analyzed for mRNA EF.
#2: We determined whether FBM involving 24-72 hrs storage at room temperature and shipping of eluates by overnight mail to our laboratory is non-inferior to CBM with respect to mRNA EF; 14 paired urine samples were tested.
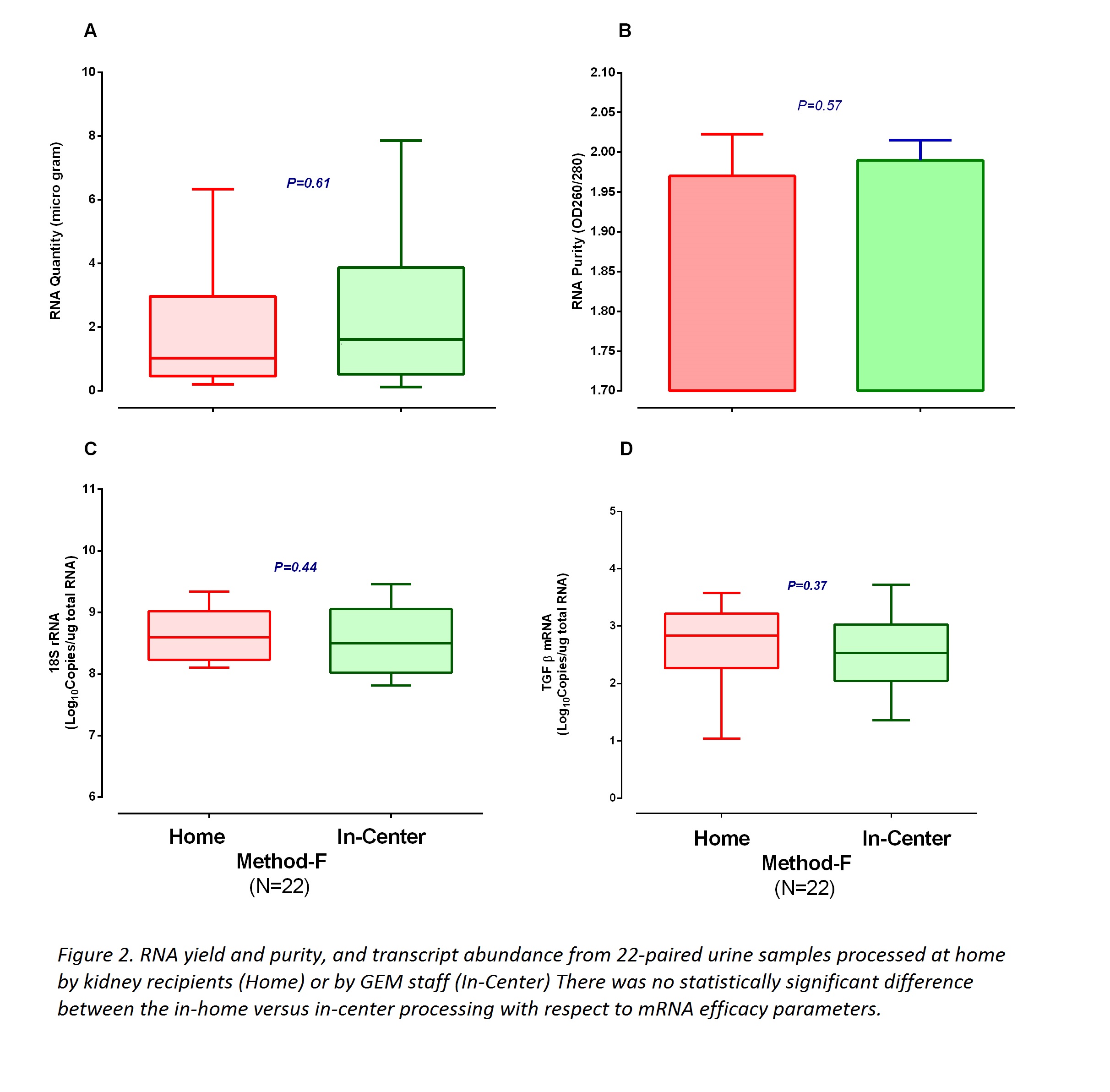
#3: Kidney recipients were trained to process urine samples by FBM. One day prior to clinic visit, they prepared filter eluates at home and brought to the clinic, and provided a fresh urine specimen for processing by our laboratory staff using FBM. Five patients and 22 paired samples were processed.
*Results: #1: mRNA efficacy parameters following processing of urine by FBM was non-inferior to CBM (Figure 1)
#2: FBM involving storage/shipping was non-inferior to CBM.
#3: FBM by kidney graft recipients was non-inferior to FBM by our laboratory staff (Figure 2).
mRNA EF were within the predetermined limits of agreement by Balnd-Altman analysis.
*Conclusions: RNA yield, purity, and transcript abundance by a filtration-based method, even when kidney allograft recipients themselves process urine at home, is non-inferior to laboratory-processed centrifugation- based protocol. Implementation of such a point-of-care approach has high potential for personalizing molecular monitoring of kidney transplant recipients.
To cite this abstract in AMA style:
Snopkowski C, Li C, Albakry S, Botticelli B, Cassidy M, Lee J, Lubetzky M, Dadhania D, Yang H, Ding R, Muthukumar T, Suthanthiran M. Efficacious Home Processing of Urine for Messenger RNA Profiling: A Point-of-Care Approach for Molecular Monitoring of Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/efficacious-home-processing-of-urine-for-messenger-rna-profiling-a-point-of-care-approach-for-molecular-monitoring-of-kidney-transplant-recipients/. Accessed March 5, 2026.« Back to 2019 American Transplant Congress