Effect of Mycophenolate Mofetil Dosing on Antibody Response to SARS-CoV-2 Vaccination in Heart and Lung Transplant Recipients
J. Mitchell1, T. P. Chiang1, J. Alejo1, A. Chang1, A. Abedon1, R. Avery1, A. Tobian1, A. Massie1, M. Levan1, D. Warren1, J. Garonzik-Wang2, D. Segev1, W. Werbel1
1Johns Hopkins University, Baltimore, MD, 2University of Wisconsin-Madison, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 243
Keywords: COVID-19, Immunosuppression, Mycophenolate mofetil, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: COVID-19 Infections Part 2: All Organs
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:30pm-3:40pm
Presentation Time: 3:30pm-3:40pm
Location: Hynes Ballroom B
*Purpose: Mycophenolate mofetil (MMF) use is associated with decreased antibody response to the SARS-CoV-2 mRNA vaccine series in heart and lung transplant recipients (HLTRs). Higher MMF doses have been associated with poor immunogenicity in kidney transplant recipients, but limited data exist on HLTRs. We evaluated the relationship between daily MMF dose and vaccine-induced antibody response in HLTRs.
*Methods: HLTRs (n= 212) from an observational cohort were categorized by daily MMF doses (None, Low: <1000mg, Moderate: 1000-2000mg, High: ≥2000mg). Semi-quantitative antibody testing was performed at 1, 3, and 6-months post-dose 2 (D2) using the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (EIA), testing for antibodies to SARS‑CoV‑2 spike protein receptor binding domain, and the EUROIMMUN EIA, testing for S1 domain of SARS-CoV-2 spike protein. Multivariable Poisson regression was used to estimate the risk of a negative antibody response with increasing MMF dose.
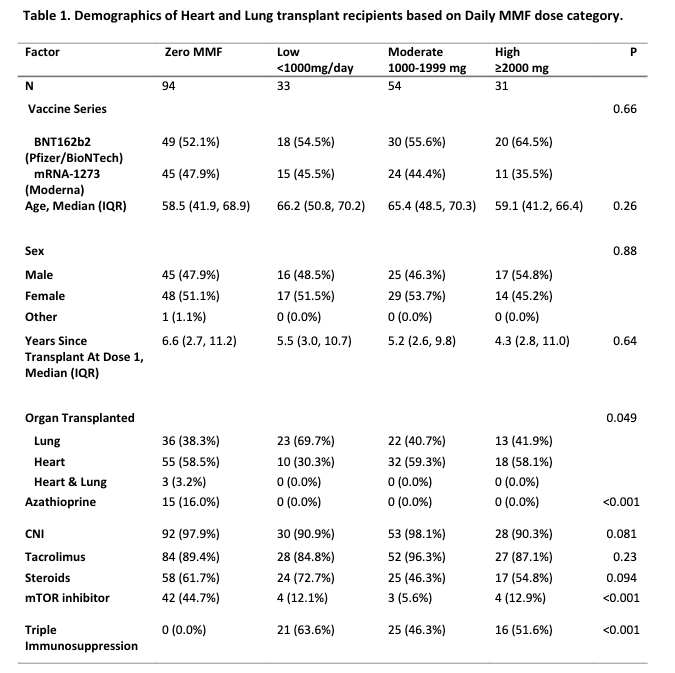
*Results: At the time of vaccination, 94 (44.3%) HLTRs reported receiving no MMF, 33 (15.6%) reported a low dose, 54 (25.7%) reported a moderate dose, and 31 (14.8%) reported a high dose regimen. There were statistically significant differences in the number of participants on mTOR inhibitors and Triple immunosuppression among the groups but the participants in all 4 dose categories were otherwise comparable (Table 1) The risk ratio of a negative post-D2 titer with low, moderate and high dose regimens compared to no MMF was 0.65 1.15 2.05 (p=0.63), 1.34 2.04 3.10 (p=0.001) and 1.83 2.77 4.21 (p<0.001) after adjusting for age, sex, vaccine type, time since transplant, and corticosteroid use.
*Conclusions: HLTRs taking MMF >1000mg/day are at higher risk of remaining seronegative after mRNA vaccination, with evidence of a dose-nonresponse effect. The findings support the exploration of whether targeted MMF reduction strategies in HLTRs increase SARS-CoV-2 vaccine immunogenicity.
To cite this abstract in AMA style:
Mitchell J, Chiang TP, Alejo J, Chang A, Abedon A, Avery R, Tobian A, Massie A, Levan M, Warren D, Garonzik-Wang J, Segev D, Werbel W. Effect of Mycophenolate Mofetil Dosing on Antibody Response to SARS-CoV-2 Vaccination in Heart and Lung Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/effect-of-mycophenolate-mofetil-dosing-on-antibody-response-to-sars-cov-2-vaccination-in-heart-and-lung-transplant-recipients/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress