Effect of Everolimus with Reduced Calcineurin Inhibitor on Efficacy and Safety in De Novo Kidney Transplant Recipients: 12-Month Results from the US Cohort of TRANSFORM Study
1TRANSFORM Study Group, Livingston
2Novartis Pharmaceuticals Corporation, East Hanover.
Meeting: 2018 American Transplant Congress
Abstract number: 35
Keywords: Efficacy, Immunosuppression, Kidney transplantation, Safety
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: mTORi Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Room 6A
Purpose: Despite remarkable short-term survival benefits, long-term outcomes in kidney transplant recipients (KTRs) remain unsatisfactory. Everolimus (EVR) may improve long-term outcomes by reducing calcineurin inhibitor (CNI)-associated nephrotoxicity. The 12-month (M) results from TRANSFORM study showed comparable efficacy and safety in EVR+reduced (r) CNI vs mycophenolic acid (MPA)+standard (s) CNI arms. Here we evaluate the efficacy and safety in the US cohort of TRANSFORM.
Methods: In this 24M, multicenter, open-label study, de novo adult KTRs were randomized to EVR+rCNI (N=1022; EVR C0: 3-8ng/mL; tacrolimus [TAC] C0: 4-7, 2-5, 2-4ng/mL; cyclosporine A [CsA] C0: 100-150, 50-100, 25-50ng/mL) or MPA+sCNI arms (N=1015; TAC C0: 8-12, 6-10, 5-8ng/mL; CsA C0: 200-300, 150-200, 100-200ng/mL, from Day1-M2, M3-M6, and M7-M24, respectively), with induction+steroids. The composite of treated biopsy-proven acute rejection (tBPAR) or eGFR <50 mL/min/1.73m2 and that of tBPAR, graft loss (GL) or death; renal function (eGFR); and safety at M12 are summarized.
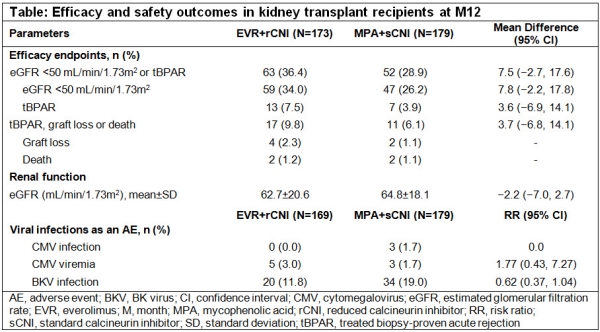
Results: Data from 173 and 179 patients in EVR+rCNI and MPA+sCNI arm was analyzed from the US cohort. Baseline characteristics were balanced between arms. Overall >90% patients received TAC. Mean EVR C0 was within the target range in EVR+rCNI arm up to M12. Mean TAC C0 was mostly above target range in EVR+rCNI and within target range in MPA+sCNI arm up to M12. Incidence of composite of eGFR <50 mL/min/1.73m2 or tBPAR was comparable. Incidence of composite of tBPAR, GL or death was low in both arms (Table).Two deaths were reported in each arm. At M12, mean eGFR in EVR+rCNI and MPA+sCNI arms was 62.7 and 64.8 mL/min/1.73m2, respectively. Viral infections were less frequent in EVR+rCNI vs MPA+sCNI arm in the overall as well as in the US population.
Conclusion: In line with the overall population, results from the US cohort of TRANSFORM study show that de novo EVR+rCNI vs MPA+sCNI provides comparable anti-rejection efficacy and renal function with less viral infections at M12.
CITATION INFORMATION: Mulgaonkar S., Qazi Y., Kim D., Peddi V., McCague K., Patel D., Wiseman A. Effect of Everolimus with Reduced Calcineurin Inhibitor on Efficacy and Safety in De Novo Kidney Transplant Recipients: 12-Month Results from the US Cohort of TRANSFORM Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Mulgaonkar S, Qazi Y, Kim D, Peddi V, McCague K, Patel D, Wiseman A. Effect of Everolimus with Reduced Calcineurin Inhibitor on Efficacy and Safety in De Novo Kidney Transplant Recipients: 12-Month Results from the US Cohort of TRANSFORM Study [abstract]. https://atcmeetingabstracts.com/abstract/effect-of-everolimus-with-reduced-calcineurin-inhibitor-on-efficacy-and-safety-in-de-novo-kidney-transplant-recipients-12-month-results-from-the-us-cohort-of-transform-study/. Accessed March 6, 2026.« Back to 2018 American Transplant Congress